Discovery and Development of a Novel mPGES-1/5-LOX Dual Inhibitor LFA-9 for Prevention and Treatment of Chronic Inflammatory Diseases
- PMID: 33408499
- PMCID: PMC7781011
- DOI: 10.2147/JIR.S286110
Discovery and Development of a Novel mPGES-1/5-LOX Dual Inhibitor LFA-9 for Prevention and Treatment of Chronic Inflammatory Diseases
Abstract
Background: Non-steroidal anti-inflammatory drugs, cyclooxygenase (COX)-2 selective inhibitors, have been explored for prevention and treatment of several inflammatory chronic conditions including arthritis, and cancer. However, the long-term use of these drugs is associated with gastrointestinal, renal, and cardiovascular side effects. Later, COX/5-lipoxygenase (5-LOX) dual inhibitors (eg, licofelone) have been developed but did not enter into the market from the clinical trails due to COX-1/2 inhibition-associated side effects. Hence, targeting microsomal prostaglandin E synthase-1 (mPGES-1) and 5-LOX can be an ideal approach while sparing COX-1/2 activities for development of the next generation of anti-inflammatory drugs with better efficacy and safety.
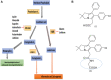
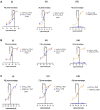
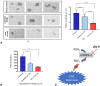
Materials and methods: In silico (molecular modelling) studies were used to design a mPGES-1/5-LOX dual inhibitory and COX-1/2 sparing lead molecule licofelone analogue-9 (LFA-9) by modifying the pharmacophore of licofelone. In vitro cell-free enzymatic (mPGES-1, 5-LOX, COX-1/2) assays using fluorometric/colorimetric methods and cell-based assays (LPS-induced PGE2, LTB4, and PGI2 productions from macrophages) using ELISA technique, isothermal calorimetry, and circular dichroism techniques were performed to determine the mPGES-1/5-LOX inhibitory efficacy and selectivity. Anti-inflammatory efficacy of LFA-9 was evaluated using a carrageenan (inflammogen)-induced rat paw edema model. Infiltration/expression of CD68 immune cells and TNF-α in paw tissues were evaluated using confocal microscope and immunoblot analysis. Anti-cancer effect of LFA-9 was evaluated using colon spheroids in vitro.
Results: LFA-9 inhibited mPGES-1/5-LOX and their products PGE2 and LTB4, spared COX-1/2 and its product PGI2. LFA-9 bound strongly with human mPGES-1/5-LOX enzymes and induced changes in their secondary structure, thereby inhibited their enzymatic activities. LFA-9 inhibited carrageenan-induced inflammation (70.4%) in rats and suppressed CD68 immune cell infiltration (P ≤ 0.0001) and TNF-α expression. LFA-9 suppressed colon tumor stemness (60.2%) in vitro through inhibition of PGE2 (82%) levels.
Conclusion: Overall study results suggest that LFA-9 is a mPGES-1/5-LOX dual inhibitor and showed anti-inflammatory and colorectal cancer preventive activities, and warranted detailed studies.
Keywords: LFA-9; anti-inflammatory agent; cancer chemoprevention; drug design; mPGES-1/5-LOX dual inhibitor.
© 2020 Yarla et al.
Conflict of interest statement
Gopal Pathuri reports a patent US10206904B2 issued and a patent WO2017/015013 pending. Hariprasad Gali reports a patent US Patent 10206904 issued. Chinthalapally V. Rao reports a patent US10206904B2 issued and a patent WO2017/015013 A1 pending. The authors report no other potential conflicts of interest for this work.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

