Identification and Functional Assessment of the First Placental Adhesin of Treponema pallidum That May Play Critical Role in Congenital Syphilis
- PMID: 33408711
- PMCID: PMC7779807
- DOI: 10.3389/fmicb.2020.621654
Identification and Functional Assessment of the First Placental Adhesin of Treponema pallidum That May Play Critical Role in Congenital Syphilis
Abstract
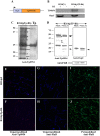
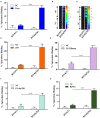
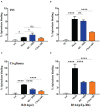
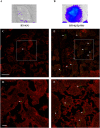
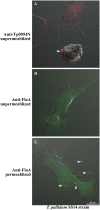
Syphilis is a global, re-emerging sexually transmitted infection and congenital syphilis remains a major cause of adverse pregnancy outcomes due to bacterial infection in developing nations with a high rate of fetus loss. The molecular mechanisms involved in pathogenesis of the causative agent, Treponema pallidum subsp. pallidum remain poorly understood due to the difficulties of working with this pathogen, including the inability to grow it in pure culture. To reduce the spread of syphilis, we must first increase our knowledge of the virulence factors of T. pallidum and their contribution to syphilis manifestations. Tp0954 was predicted to be a surface lipoprotein of T. pallidum. Therefore, we experimentally demonstrated that Tp0954 is indeed a surface protein and further investigated its role in mediating bacterial attachment to various mammalian host cells. We found that expression of Tp0954 in a poorly adherent, but physiologically related derivative strain of the Lyme disease causing spirochete Borrelia burgdorferi B314 strain promotes its binding to epithelial as well as non-epithelial cells including glioma and placental cell lines. We also found that Tp0954 expression facilitates binding of this strain to purified dermatan sulfate and heparin, and also that bacterial binding to mammalian cell lines is mediated by the presence of heparan sulfate and dermatan sulfate in the extracellular matrix of the specific cell lines. These results suggest that Tp0954 may be involved not only in initiating T. pallidum infection by colonizing skin epithelium, but it may also contribute to disseminated infection and colonization of distal tissues. Significantly, we found that Tp0954 promotes binding to the human placental choriocarcinoma BeWo cell line, which is of trophoblastic endocrine cell type, as well as human placental tissue sections, suggesting its role in placental colonization and possible contribution to transplacental transmission of T. pallidum. Altogether, these novel findings offer an important step toward unraveling syphilis pathogenesis, including placental colonization and T. pallidum vertical transmission from mother to fetus during pregnancy.
Keywords: Borrelia burgdorferi; Tp0954; Treponema pallidum subspecies pallidum; congenital syphilis; heterologous expression system; placenta binding adhesin; surrogate system.
Copyright © 2020 Primus, Rocha, Giacani and Parveen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ayres Pereira M., Mandel Clausen T., Pehrson C., Mao Y., Resende M., Daugaard M., et al. (2016). Placental sequestration of Plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and chondroitin sulfate a on syndecan-1. PLoS Pathog. 12:e1005831. 10.1371/journal.ppat.1005831 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

