The 2020 race towards SARS-CoV-2 specific vaccines
- PMID: 33408775
- PMCID: PMC7778607
- DOI: 10.7150/thno.53691
The 2020 race towards SARS-CoV-2 specific vaccines
Abstract
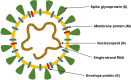
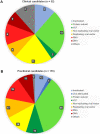
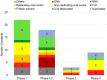
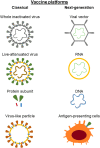
The global outbreak of a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) highlighted a requirement for two pronged clinical interventions such as development of effective vaccines and acute therapeutic options for medium-to-severe stages of "coronavirus disease 2019" (COVID-19). Effective vaccines, if successfully developed, have been emphasized to become the most effective strategy in the global fight against the COVID-19 pandemic. Basic research advances in biotechnology and genetic engineering have already provided excellent progress and groundbreaking new discoveries in the field of the coronavirus biology and its epidemiology. In particular, for the vaccine development the advances in characterization of a capsid structure and identification of its antigens that can become targets for new vaccines. The development of the experimental vaccines requires a plethora of molecular techniques as well as strict compliance with safety procedures. The research and clinical data integrity, cross-validation of the results, and appropriated studies from the perspective of efficacy and potently side effects have recently become a hotly discussed topic. In this review, we present an update on latest advances and progress in an ongoing race to develop 52 different vaccines against SARS-CoV-2. Our analysis is focused on registered clinical trials (current as of November 04, 2020) that fulfill the international safety and efficacy criteria in the vaccine development. The requirements as well as benefits and risks of diverse types of SARS-CoV-2 vaccines are discussed including those containing whole-virus and live-attenuated vaccines, subunit vaccines, mRNA vaccines, DNA vaccines, live vector vaccines, and also plant-based vaccine formulation containing coronavirus-like particle (VLP). The challenges associated with the vaccine development as well as its distribution, safety and long-term effectiveness have also been highlighted and discussed.
Keywords: COVID-19; SARS-CoV-2; clinical trials; coronavirus disease 2019; severe acute respiratory syndrome coronavirus 2; vaccine.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Zeidler A, Karpiński TM. What do we know about SARS-CoV-2 virus and COVID-19 disease? J Pre-Clin Clin Res. 2020;14:33–8.
-
- COVID-19 situation update worldwide, as of 7 August 2020. European Centre for Disease Prevention and Control. Available at: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases.
-
- EU Clinical Trials Register - Update. Available at: https://www.clinicaltrialsregister.eu/
-
- Clinical Trials Registry - India (CTRI). Available at: http://ctri.nic.in/Clinicaltrials/login.php.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

