Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance
- PMID: 33408785
- PMCID: PMC7778599
- DOI: 10.7150/thno.50905
Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance
Abstract
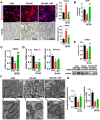
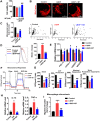
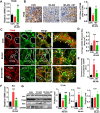
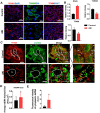
Aims: Ischemia-reperfusion injury (IRI)-induced acute kidney injury (IRI-AKI) is characterized by elevated levels of reactive oxygen species (ROS), mitochondrial dysfunction, and inflammation, but the potential link among these features remains unclear. In this study, we aimed to investigate the specific role of mitochondrial ROS (mtROS) in initiating mitochondrial DNA (mtDNA) damage and inflammation during IRI-AKI. Methods: The changes in renal function, mitochondrial function, and inflammation in IRI-AKI mice with or without mtROS inhibition were analyzed in vivo. The impact of mtROS on TFAM (mitochondrial transcription factor A), Lon protease, mtDNA, mitochondrial respiration, and cytokine release was analyzed in renal tubular cells in vitro. The effects of TFAM knockdown on mtDNA, mitochondrial function, and cytokine release were also analyzed in vitro. Finally, changes in TFAM and mtDNA nucleoids were measured in kidney samples from IRI-AKI mice and patients. Results: Decreasing mtROS levels attenuated renal dysfunction, mitochondrial damage, and inflammation in IRI-AKI mice. Decreasing mtROS levels also reversed the decrease in TFAM levels and mtDNA copy number that occurs in HK2 cells under oxidative stress. mtROS reduced the abundance of mitochondrial TFAM in HK2 cells by suppressing its transcription and promoting Lon-mediated TFAM degradation. Silencing of TFAM abolished the Mito-Tempo (MT)-induced rescue of mitochondrial function and cytokine release in HK2 cells under oxidative stress. Loss of TFAM and mtDNA damage were found in kidneys from IRI-AKI mice and AKI patients. Conclusion: mtROS can promote renal injury by suppressing TFAM-mediated mtDNA maintenance, resulting in decreased mitochondrial energy metabolism and increased cytokine release. TFAM defects may be a promising target for renal repair after IRI-AKI.
Keywords: ROS; TFAM; acute kidney injury; mitochondria; mtDNA.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






Similar articles
-
Upregulation of Mitochondrial Transcription Factor A Promotes the Repairment of Renal Tubular Epithelial Cells in Sepsis by Inhibiting Reactive Oxygen Species-Mediated Toll-Like Receptor 4/p38MAPK Signaling.Pathobiology. 2019;86(5-6):263-273. doi: 10.1159/000501789. Epub 2019 Aug 20. Pathobiology. 2019. PMID: 31430762
-
Control release of mitochondria-targeted antioxidant by injectable self-assembling peptide hydrogel ameliorated persistent mitochondrial dysfunction and inflammation after acute kidney injury.Drug Deliv. 2018 Nov;25(1):546-554. doi: 10.1080/10717544.2018.1440445. Drug Deliv. 2018. PMID: 29451033 Free PMC article.
-
The interaction between C/EBPβ and TFAM promotes acute kidney injury via regulating NLRP3 inflammasome-mediated pyroptosis.Mol Immunol. 2020 Nov;127:136-145. doi: 10.1016/j.molimm.2020.08.023. Epub 2020 Sep 21. Mol Immunol. 2020. PMID: 32971400
-
Mitochondrial metabolism and targeted treatment strategies in ischemic-induced acute kidney injury.Cell Death Discov. 2024 Feb 10;10(1):69. doi: 10.1038/s41420-024-01843-5. Cell Death Discov. 2024. PMID: 38341438 Free PMC article. Review.
-
The mitochondrial transcription factor TFAM in neurodegeneration: emerging evidence and mechanisms.FEBS Lett. 2018 Mar;592(5):793-811. doi: 10.1002/1873-3468.12989. Epub 2018 Feb 15. FEBS Lett. 2018. PMID: 29364506 Free PMC article. Review.
Cited by
-
Melatonin attenuates liver ischemia-reperfusion injury via inhibiting the PGAM5-mPTP pathway.PLoS One. 2024 Oct 29;19(10):e0312853. doi: 10.1371/journal.pone.0312853. eCollection 2024. PLoS One. 2024. PMID: 39471139 Free PMC article.
-
SETDB1 targeting SESN2 regulates mitochondrial damage and oxidative stress in renal ischemia-reperfusion injury.BMC Biol. 2024 Oct 23;22(1):246. doi: 10.1186/s12915-024-02048-z. BMC Biol. 2024. PMID: 39443993 Free PMC article.
-
LIGHT deficiency attenuates acute kidney disease development in an in vivo experimental renal ischemia and reperfusion injury model.Cell Death Discov. 2022 Sep 26;8(1):399. doi: 10.1038/s41420-022-01188-x. Cell Death Discov. 2022. PMID: 36163116 Free PMC article.
-
Mitochondria-Targeted Antioxidant Mitoquinone Maintains Mitochondrial Homeostasis through the Sirt3-Dependent Pathway to Mitigate Oxidative Damage Caused by Renal Ischemia/Reperfusion.Oxid Med Cell Longev. 2022 Sep 20;2022:2213503. doi: 10.1155/2022/2213503. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36193071 Free PMC article.
-
Cellular senescence in ischemia/reperfusion injury.Cell Death Discov. 2022 Oct 18;8(1):420. doi: 10.1038/s41420-022-01205-z. Cell Death Discov. 2022. PMID: 36253355 Free PMC article. Review.
References
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

