In Vitro Analysis of Histology, Mechanics, and Safety of Radiation-free Pre-hydrated Human Acellular Dermal Matrix
- PMID: 33408889
- PMCID: PMC7779726
- DOI: 10.4048/jbc.2020.23.e64
In Vitro Analysis of Histology, Mechanics, and Safety of Radiation-free Pre-hydrated Human Acellular Dermal Matrix
Abstract
Purpose: Acellular dermal matrix (ADM) supports tissue expanders or implants in implant-based breast reconstruction. The characteristics of ADM tissue are defined by the manufacturing procedure, such as decellularization, preservation, and sterilization, and are directly related to clinical outcomes. This study aimed to compare the properties of a new pre-hydrated-ADM (H-ADM-low) obtained using a decellularization reagent reduction process with a low concentration of detergent with those of radiation-sterilized H-ADM and freeze-dried ADM (FD-ADM).
Methods: ADMs were evaluated in terms of structure, mechanical quality, and cytotoxicity using histochemical staining, tensile strength testing, and in vitro cell viability analysis.
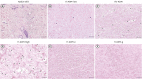
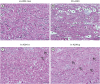
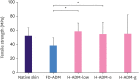
Results: The tissue structure of H-ADM-low (CGDERM ONE-STEP) was similar to that of native skin despite complete decellularization. By contrast, in FD-ADM, the tissue structure was damaged by the freeze-drying process, and radiation-sterilized H-ADM showed a compact fibrillar arrangement. Furthermore, matrix components such as collagen and elastin were preserved in H-ADM-low, whereas a loss of elastin fibers with fragmented distribution was observed in radiation-sterilized H-ADMs. H-ADM-low's tensile strength (58.84 MPa) was significantly greater than that of FD-ADM (38.60 MPa) and comparable with that of radiation-sterilized H-ADMs. The residual detergent content in H-ADM-low (47.45 mg/L) was 2.67-fold lower than that of H-ADM decellularized with a conventional detergent concentration (126.99 mg/mL), and this finding was consistent with the cell viability results (90.7% and 70.7%, respectively), indicating that H-ADM-low has very low cytotoxicity.
Conclusions: H-ADM-low produced through aseptic processes retains the original tissue structure, demonstrates excellent mechanical properties, and does not affect cell viability. Therefore, this newer H-ADM is suitable for use in implant-based breast reconstruction.
Keywords: Acellular dermis; Biological preservation; Breast implantation; Detergents; In vitro techniques.
© 2020 Korean Breast Cancer Society.
Conflict of interest statement
Conflict of Interest: The authors declare that they have no competing interests.
Figures



References
-
- Capito AE, Tholpady SS, Agrawal H, Drake DB, Katz AJ. Evaluation of host tissue integration, revascularization, and cellular infiltration within various dermal substrates. Ann Plast Surg. 2012;68:495–500. - PubMed
-
- Garcia O, Jr, Scott JR. Analysis of acellular dermal matrix integration and revascularization following tissue expander breast reconstruction in a clinically relevant large-animal model. Plast Reconstr Surg. 2013;131:741e–751e. - PubMed
-
- Bellows CF, Alder A, Helton WS. Abdominal wall reconstruction using biological tissue grafts: present status and future opportunities. Expert Rev Med Devices. 2006;3:657–675. - PubMed
-
- Galili U. The α-Gal epitope (Galα1-3Galβ1-4GlcNAc-R) in xenotransplantation. Biochimie. 2001;83:557–563. - PubMed
-
- Armour AD, Fish JS, Woodhouse KA, Semple JL. A comparison of human and porcine acellularized dermis: interactions with human fibroblasts in vitro . Plast Reconstr Surg. 2006;117:845–856. - PubMed
LinkOut - more resources
Full Text Sources

