Quantitative multi-contrast in vivo mouse imaging with polarization diversity optical coherence tomography and angiography
- PMID: 33408972
- PMCID: PMC7747897
- DOI: 10.1364/BOE.403209
Quantitative multi-contrast in vivo mouse imaging with polarization diversity optical coherence tomography and angiography
Abstract
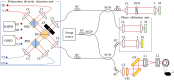
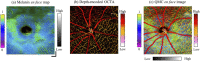
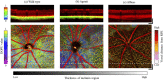
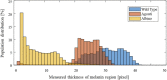
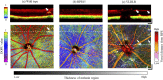
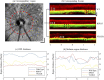
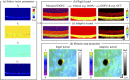
Retinal microvasculature and the retinal pigment epithelium (RPE) play vital roles in maintaining the health and metabolic activity of the eye. Visualization of these retina structures is essential for pre-clinical studies of vision-robbing diseases, such as age-related macular degeneration (AMD). We have developed a quantitative multi-contrast polarization diversity OCT and angiography (QMC-PD-OCTA) system for imaging and visualizing pigment in the RPE using degree of polarization uniformity (DOPU), along with flow in the retinal capillaries using OCT angiography (OCTA). An adaptive DOPU averaging kernel was developed to increase quantifiable values from visual data, and QMC en face images permit simultaneous visualization of vessel location, depth, melanin region thickness, and mean DOPU values, allowing rapid identification and differentiation of disease symptoms. The retina of five different mice strains were measured in vivo, with results demonstrating potential for pre-clinical studies of retinal disorders.
© 2020 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
MVS: Seymour Vision (I). SM, YY: Yokogawa Electric Corp. (F), Nikon (F), Kao Corp. (F), Topcon (F), Tomey Corp (F, P), Sky Technology (F).
Figures



References
-
- Huber G., Beck S. C., Grimm C., Sahaboglu-Tekgoz A., Paquet-Durand F., Wenzel A., Humphries P., Michael Redmond T., Seeliger M. W., Dominik Fischer M., “Spectral domain optical coherence tomography in mouse models of retinal degeneration,” Invest. Ophthalmol. Visual Sci. 50(12), 5888–5895 (2009). 10.1167/iovs.09-3724 - DOI - PMC - PubMed
-
- Zhang P., Zam A., Jian Y., Wang X., Li Y., Lam K. S., Burns M. E., Sarunic M. V., Pugh E. N., Zawadzki R. J., “In vivo wide-field multispectral scanning laser ophthalmoscopy–optical coherence tomography mouse retinal imager: longitudinal imaging of ganglion cells, microglia, and Müller glia, and mapping of the mouse retinal and choroidal vasculature,” J. Biomed. Opt. 20(12), 126005 (2015). 10.1117/1.JBO.20.12.126005 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
