Wearable device for remote monitoring of transcutaneous tissue oxygenation
- PMID: 33408975
- PMCID: PMC7747925
- DOI: 10.1364/BOE.408850
Wearable device for remote monitoring of transcutaneous tissue oxygenation
Abstract
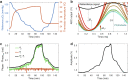
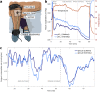
Wearable devices have found widespread applications in recent years as both medical devices as well as consumer electronics for sports and health tracking. A metric of health that is often overlooked in currently available technology is the direct measurement of molecular oxygen in living tissue, a key component in cellular energy production. Here, we report on the development of a wireless wearable prototype for transcutaneous oxygenation monitoring based on quantifying the oxygen-dependent phosphorescence of a metalloporphyrin embedded within a highly breathable oxygen sensing film. The device is completely self-contained, weighs under 30 grams, performs on-board signal analysis, and can communicate with computers or smartphones. The wearable measures tissue oxygenation at the skin surface by detecting the lifetime and intensity of phosphorescence, which undergoes quenching in the presence of oxygen. As well as being insensitive to motion artifacts, it offers robust and reliable measurements even in variable atmospheric conditions related to temperature and humidity. Preliminary in vivo testing in a porcine ischemia model shows that the wearable is highly sensitive to changes in tissue oxygenation in the physiological range upon inducing a decrease in limb perfusion.
© 2020 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Butler M. S., Luebbers P. E., “Health and fitness wearables,” in Wearable Technologies: Concepts, Methodologies, Tools, and Applications, (IGI Global, 2018), pp. 30–50.
LinkOut - more resources
Full Text Sources
Research Materials
