Enteroadsorbent Polymethylsiloxane Polyhydrate vs. Probiotic Lactobacillus reuteri DSM 17938 in the Treatment of Rotaviral Gastroenteritis in Infants and Toddlers, a Randomized Controlled Trial
- PMID: 33409259
- PMCID: PMC7781153
- DOI: 10.3389/fped.2020.553960
Enteroadsorbent Polymethylsiloxane Polyhydrate vs. Probiotic Lactobacillus reuteri DSM 17938 in the Treatment of Rotaviral Gastroenteritis in Infants and Toddlers, a Randomized Controlled Trial
Abstract
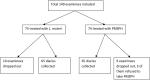
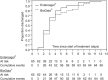
Purpose: The aim of this study was to compare two adjunct therapies in the treatment of childhood rotavirus gastroenteritis (RVGE). We compared the recommended treatment, probiotic Lactobacillus reuteri DSM 17938 (BioGaia®), vs. a novel treatment, enterosorbent polymethylsiloxane polyhydrate (Enterosgel®). Methods: This was an open-label, randomized, clinical controlled trial at the University Hospital for Infectious Diseases (UHID) in Zagreb, Croatia. A total of 149 children aged 6-36 months with acute rotaviral gastroenteritis over a period of <48 h, with no significant chronic comorbidity, were randomized to receive the standard therapy with L. reuteri DSM 17938 (hereafter L. reuteri) or polymethylsiloxane polyhydrate (hereafter PMSPH) therapy, during 5 days. The primary end point was time to recovery in days in both groups. The recovery was defined as absence of fever and vomiting and either the first firm stool, absence of stool for more than 24 h, or return of usual bowel habit. Results: A total of 75 children were randomized into the L. reuteri group and 74 were randomized into the PMSPH group; after excluding missing data, the data from 65 children in each group were analyzed. There was no significant difference in the treatment efficacy between the two regimens with an estimated median time of recovery of 6 days in both groups (p = 0.754). No significant side effects were observed in either group. Conclusion: Novel enterosorbent PMSPH had a similar efficacy to probiotic L. reuteri in the treatment of rotaviral gastroenteritis in preschool children. Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT04116307 [October 3, 2019] (retrospectively registered). https://clinicaltrials.gov/show/NCT04116307.
Keywords: Lactobacillus reuteri DSM 17938; children; gastroenteritis; polymethylsiloxane polyhidrate; rotavirus.
Copyright © 2020 Markovinović, Knezović, Kniewald, Stemberger Marić, Trkulja and Tešović.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization Diarrhoeal Disease. (2017). Available online at: http://www.who.int/mediacentre/factsheets/fs330/en/ (accessed April 12, 2018).
-
- Croatian Institute of Public Health Implementation of Immunization, Immunoprophylaxis and Chemoprophylaxis Program in Croatia in 2017 (2017). Available online at: https://www.hzjz.hr/wp-content/uploads/2017/05/PROVEDBENI-PROGRAM-II._20... (accessed November 20, 2020).
-
- Dormitzer PR. Rotavirus. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett's: Principles and Practice of Infectious Diseases. 9th ed Philadelphia, PA: Churchill Livingstone; (2020). p. 1983–96.
-
- Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. (1991) 88:90–7. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

