Reversal of Endothelial Extracellular Vesicle-Induced Smooth Muscle Phenotype Transition by Hypercholesterolemia Stimulation: Role of NLRP3 Inflammasome Activation
- PMID: 33409276
- PMCID: PMC7779768
- DOI: 10.3389/fcell.2020.597423
Reversal of Endothelial Extracellular Vesicle-Induced Smooth Muscle Phenotype Transition by Hypercholesterolemia Stimulation: Role of NLRP3 Inflammasome Activation
Abstract
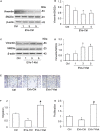
Recent studies reported that vascular endothelial cells (ECs) secrete NLR family pyrin domain-containing 3 (NLRP3) inflammasome products such as interleukin-1β (IL-1β) via extracellular vesicles (EVs) under various pathological conditions. EVs represent one of the critical mechanisms mediating the cell-to-cell communication between ECs and vascular smooth muscle cells (VSMCs). However, whether or not the inflammasome-dependent EVs directly participate in the regulation of VSMC function remains unknown. In the present study, we found that in cultured carotid ECs, atherogenic stimulation by oxysterol 7-ketocholesterol (7-Ket) induced NLRP3 inflammasome formation and activation, reduced lysosome-multivesicular bodies (MVBs) fusion, and increased secretion of EVs that contain inflammasome product IL-1β. These EC-derived IL-1β-containing EVs promoted synthetic phenotype transition of co-cultured VSMCs, whereas EVs from unstimulated ECs have the opposite effects. Moreover, acid ceramidase (AC) deficiency or lysosome inhibition further exaggerated the 7-Ket-induced release of IL-1β-containing EVs in ECs. Using a Western diet (WD)-induced hypercholesterolemia mouse model, we found that endothelial-specific AC gene knockout mice (Asah1fl/fl/ECCre) exhibited augmented WD-induced EV secretion with IL-1β and more significantly decreased the interaction of MVBs with lysosomes in the carotid arterial wall compared to their wild-type littermates (WT/WT). The endothelial AC deficiency in Asah1fl/fl/ECCre mice also resulted in enhanced VSMC phenotype transition and accelerated neointima formation. Together, these results suggest that NLRP3 inflammasome-dependent IL-1β production during hypercholesterolemia promotes VSMC phenotype transition to synthetic status via EV machinery, which is controlled by lysosomal AC activity. Our findings provide novel mechanistic insights into understanding the pathogenic role of endothelial NLRP3 inflammasome in vascular injury through EV-mediated EC-to-VSMC regulation.
Keywords: acid ceramidase; ceramide; endothelial cells; extracellular vesicles; lysosome.
Copyright © 2020 Yuan, Bhat, Samidurai, Das, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Adhikari N., Shekar K. C., Staggs R., Win Z., Steucke K., Lin Y. W., et al. (2015). Guidelines for the isolation and characterization of murine vascular smooth muscle cells. A report from the international society of cardiovascular translational research. J. Cardiovasc. Transl. Res. 8 158–163. 10.1007/s12265-015-9616-6 - DOI - PMC - PubMed
-
- Arriola Benitez P. C., Pesce Viglietti A. I., Gomes M. T. R., Oliveira S. C., Quarleri J. F., Giambartolomei G. H., et al. (2019). Brucella abortus infection elicited hepatic stellate cell-mediated fibrosis through inflammasome-dependent IL-1beta production. Front. Immunol. 10:3036 10.3389/fimmu.2019.03036 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

