Orai channels: key players in Ca2+ homeostasis
- PMID: 33409426
- PMCID: PMC7116546
- DOI: 10.1016/j.cophys.2020.06.006
Orai channels: key players in Ca2+ homeostasis
Abstract
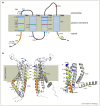
Maintaining a precise calcium (Ca2+) balance is vital for cellular survival. The most prominent pathway to shuttle Ca2+ into cells is the Ca2+ release activated Ca2+ (CRAC) channel. Orai proteins are indispensable players in this central mechanism of Ca2+ entry. This short review traces the latest articles published in the field of CRAC channel signalling with a focus on the structure of the pore-forming Orai proteins, the propagation of the binding signal from STIM1 through the channel to the central pore and their role in human health and disease.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures
References
-
- Feske S, et al. Amutation inOrai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
