Structural aspects of the aging invertebrate brain
- PMID: 33409654
- PMCID: PMC7965346
- DOI: 10.1007/s00441-020-03314-6
Structural aspects of the aging invertebrate brain
Abstract
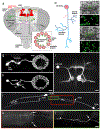
Aging is characterized by a decline in neuronal function in all animal species investigated so far. Functional changes are accompanied by and may be in part caused by, structurally visible degenerative changes in neurons. In the mammalian brain, normal aging shows abnormalities in dendrites and axons, as well as ultrastructural changes in synapses, rather than global neuron loss. The analysis of the structural features of aging neurons, as well as their causal link to molecular mechanisms on the one hand, and the functional decline on the other hand is crucial in order to understand the aging process in the brain. Invertebrate model organisms like Drosophila and C. elegans offer the opportunity to apply a forward genetic approach to the analysis of aging. In the present review, we aim to summarize findings concerning abnormalities in morphology and ultrastructure in invertebrate brains during normal aging and compare them to what is known for the mammalian brain. It becomes clear that despite of their considerably shorter life span, invertebrates display several age-related changes very similar to the mammalian condition, including the retraction of dendritic and axonal branches at specific locations, changes in synaptic density and increased accumulation of presynaptic protein complexes. We anticipate that continued research efforts in invertebrate systems will significantly contribute to reveal (and possibly manipulate) the molecular/cellular pathways leading to neuronal aging in the mammalian brain.
Keywords: Axons; Dendrites; Invertebrates; Neurons; Normal aging; Synapses.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

