Reparative System Arising from CCR2(+) Monocyte Conversion Attenuates Neuroinflammation Following Ischemic Stroke
- PMID: 33409730
- PMCID: PMC8421302
- DOI: 10.1007/s12975-020-00878-x
Reparative System Arising from CCR2(+) Monocyte Conversion Attenuates Neuroinflammation Following Ischemic Stroke
Abstract
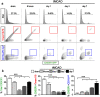
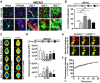
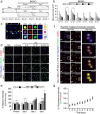
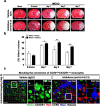
Monocytes recruitment from the blood to inflamed tissues following ischemic stroke is an important immune response to wound healing and tissue repair. Mouse monocytes can be endogenously divided into two distinct populations: pro-inflammatory or classical monocytes that express CCR2highCX3CR1low and circulate in blood, and anti-inflammatory or non-classical monocytes that express CCR2lowCX3CR1high and patrol locally. In this study of transgenic mice with functional CX3CR1GFP/+ or CX3CR1GFP/+-CCR2RFP/+, we found that CCR2highCX3CR1low monocytes recruited to the injured brain were cytokine-dependently converted into CCR2lowCX3CR1high macrophages, especially under the influence of IL-4 and IL-13, thereby attenuating the neuroinflammation following sterile ischemic stroke. The overall data suggest that (1) the regulation of monocyte-switching is one of the ultimate reparative strategies in ischemic stroke, and (2) the adaptation of monocytes in a locally inflamed milieu is vital to alleviating the effects of ischemic stroke through innate immunity.
Keywords: CCR2; CX3CR1; Macrophages; Monocytes conversion; Neuroinflammation ischemic stroke.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
