Liquid Poly-N-acetyl Glucosamine (sNAG) Improves Achilles Tendon Healing in a Rat Model
- PMID: 33409852
- PMCID: PMC8178587
- DOI: 10.1007/s10439-020-02711-w
Liquid Poly-N-acetyl Glucosamine (sNAG) Improves Achilles Tendon Healing in a Rat Model
Abstract
The Achilles tendon, while the strongest and largest tendon in the body, is frequently injured. Even after surgical repair, patients risk re-rupture and long-term deficits in function. Poly-N-acetyl glucosamine (sNAG) polymer has been shown to increase the rate of healing of venous leg ulcers, and use of this material improved tendon-to-bone healing in a rat model of rotator cuff injury. Therefore, the purpose of this study was to investigate the healing properties of liquid sNAG polymer suspension in a rat partial Achilles tear model. We hypothesized that repeated sNAG injections throughout healing would improve Achilles tendon healing as measured by improved mechanical properties and cellular morphology compared to controls. Results demonstrate that sNAG has a positive effect on rat Achilles tendon healing at three weeks after a full thickness, partial width injury. sNAG treatment led to increased quasistatic tendon stiffness, and increased tangent and secant stiffness throughout fatigue cycling protocols. Increased dynamic modulus also suggests improved viscoelastic properties with sNAG treatment. No differences were identified in histological properties. Importantly, use of this material did not have any negative effects on any measured parameter. These results support further study of this material as a minimally invasive treatment modality for tendon healing.
Keywords: Animal model; Biomechanical properties; Foot and ankle; Injury; Orthopaedics.
Figures
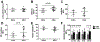
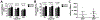
References
-
- Barfod KW, Bencke J, Lauridsen HB, Ban I, Ebskov L, and Troelsen A. Nonoperative dynamic treatment of acute Achilles tendon rupture: the influence of early weight-bearing on clinical outcome: a blinded, randomized controlled trial. J Bone Joint Surg Am. 96(18):1497–1503, 2014. - PubMed
-
- Barg A, and Ludwig T. Surgical Strategies for the Treatment of Insertional Achilles Tendinopathy. Foot Ankle Clin. 24(3):533–559, 2019. - PubMed
-
- Favata M Thesis Dissertation. Scarless healing in the fetus: implications and strategies for postnatal tendon repair. University of Pennsylvania; Philadelphia: 2006.
-
- Freedman BR, Gordon JA, Bhatt PR, Pardes AM, Thomas SJ, Sarver JJ, Riggin CN, Tucker JJ, Williams AW, Zanes RC, Hast MW, Farber DC, Silbernagel KG, and Soslowsky LJ. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. J Orthop Res 34:2172–2180, 2016. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

