Safety and Tolerability of Subcutaneous IgPro20 at High Infusion Parameters in Patients with Primary Immunodeficiency: Findings from the Pump-Assisted Administration Cohorts of the HILO Study
- PMID: 33409867
- PMCID: PMC7858210
- DOI: 10.1007/s10875-020-00912-5
Safety and Tolerability of Subcutaneous IgPro20 at High Infusion Parameters in Patients with Primary Immunodeficiency: Findings from the Pump-Assisted Administration Cohorts of the HILO Study
Abstract
Purpose: To evaluate the safety and tolerability of subcutaneous IgPro20 (Hizentra®, CSL Behring, King of Prussia, PA, USA) administered at high infusion parameters (> 25 mL and > 25 mL/h per injection site) in patients with primary immunodeficiency.
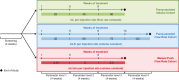
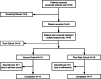
Methods: The Hizentra® Label Optimization (HILO) study was an open-label, parallel-arm, non-randomized study (NCT03033745) of IgPro20 using a forced upward titration design for infusion parameters. Patients experienced with pump-assisted IgPro20 infusions received weekly IgPro20 infusions at a stable dose in the Pump-Assisted Volume Cohort (N = 15; 25-50 mL per injection site) and in the Pump-Assisted Flow Rate Cohort (N = 18; 25-100 mL/h per injection site). Responder rates (percentage of patients who successfully completed ≥ 75% of planned infusions), safety outcomes, and serum immunoglobulin G (IgG) trough levels were evaluated.
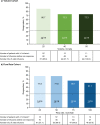
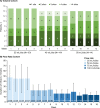
Results: Responder rates were 86.7% (13/15, 25 mL) and 73.3% (11/15, 40 and 50 mL) in the Volume Cohort, and 77.8% (14/18, 25 and 50 mL/h), 66.7% (12/18, 75 mL/h), and 61.1% (11/18, 100 mL/h) in the Flow Rate Cohort. Infusion compliance was ≥ 90% in all patients in the Volume Cohort and in 83.3% of patients in the Flow Rate Cohort. The number of injection sites (Volume Cohort) and the infusion duration (Flow Rate Cohort) decreased with increasing infusion parameters. The rate of treatment-emergent adverse events per infusion was low (0.138 [Volume Cohort] and 0.216 [Flow Rate Cohort]). Serum IgG levels remained stable during the study.
Conclusion: Pump-assisted IgPro20 infusions are feasible at 50 mL and 100 mL/h per injection site in treatment-experienced patients, which may result in fewer injection sites and shorter infusion times.
Trial registration: NCT03033745 ; registered January 27, 2017.
Keywords: IgPro20; Primary immunodeficiency (PID); high infusion flow rate; high infusion volume; pump-assisted infusion; subcutaneous Ig (SCIG).
Conflict of interest statement
JTA has served as a speaker, consultant, and clinical research principal site investigator for CSL Behring and Takeda. VRB has served as a speaker for CSL Behring and Takeda. JC has received research funding from CSL Behring and Octapharma and is a clinical trial site investigator for Takeda. CH has nothing to disclose. SSM has served as a speaker for CSL Behring, Genentech, Teva, AstraZeneca, and Regeneron and received research funding from CSL Behring, Shire, and Regeneron. NCP has served as a speaker for CSL Behring and Takeda, received research funding from CSL Behring and Takeda, and has served as an advisory board member for Horizon Therapeutics. JMR has served as a principal site investigator for CSL Behring and an independent contractor for Takeda. PS received a publication research grant from CSL Behring. DCV has received research funding from the Fonds de la recherche en santé du Quebec, Canadian Institutes of Health Research, the US Department of Defense, the Jeffrey Modell Foundation, La Fondation du Grand Défi Pierre Lavoie, and CSL Behring; has served as a speaker for CSL Behring and Avir Pharma; and has received clinical trial funding from CSL Behring and Cidara Therapeutics. JHH, MP, and MAR are employees of CSL Behring and own CSL Behring shares.
Figures
References
-
- Al-Herz W, Bousfiha A, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2011;2:54. doi: 10.3389/fimmu.2011.00054. - DOI - PMC - PubMed
-
- Jolles S, Bernatowska E, de Gracia J, Borte M, Cristea V, Peter HH, Belohradsky BH, Wahn V, Neufang-Hüber J, Zenker O, Grimbacher B. Efficacy and safety of Hizentra((R)) in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol. 2011;141(1):90–102. doi: 10.1016/j.clim.2011.06.002. - DOI - PubMed
-
- Borte M, Pac M, Serban M, Gonzalez-Quevedo T, Grimbacher B, Jolles S, et al. Efficacy and safety of Hizentra(R), a new 20% immunoglobulin preparation for subcutaneous administration, in pediatric patients with primary immunodeficiency. J Clin Immunol. 2011;31(5):752–761. doi: 10.1007/s10875-011-9557-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

