Decreased parenchymal arteriolar tone uncouples vessel-to-neuronal communication in a mouse model of vascular cognitive impairment
- PMID: 33410092
- PMCID: PMC8190257
- DOI: 10.1007/s11357-020-00305-x
Decreased parenchymal arteriolar tone uncouples vessel-to-neuronal communication in a mouse model of vascular cognitive impairment
Abstract
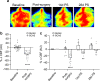
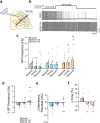
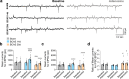
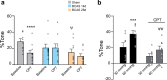
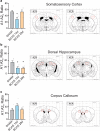
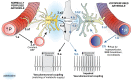
Chronic hypoperfusion is a key contributor to cognitive decline and neurodegenerative conditions, but the cellular mechanisms remain ill-defined. Using a multidisciplinary approach, we sought to elucidate chronic hypoperfusion-evoked functional changes at the neurovascular unit. We used bilateral common carotid artery stenosis (BCAS), a well-established model of vascular cognitive impairment, combined with an ex vivo preparation that allows pressurization of parenchymal arterioles in a brain slice. Our results demonstrate that mild (~ 30%), chronic hypoperfusion significantly altered the functional integrity of the cortical neurovascular unit. Although pial cerebral perfusion recovered over time, parenchymal arterioles progressively lost tone, exhibiting significant reductions by day 28 post-surgery. We provide supportive evidence for reduced adenosine 1 receptor-mediated vasoconstriction as a potential mechanism in the adaptive response underlying the reduced baseline tone in parenchymal arterioles. In addition, we show that in response to the neuromodulator adenosine, the action potential frequency of cortical pyramidal neurons was significantly reduced in all groups. However, a significant decrease in adenosine-induced hyperpolarization was observed in BCAS 14 days. At the microvascular level, constriction-induced inhibition of pyramidal neurons was significantly compromised in BCAS mice. Collectively, these results suggest that BCAS uncouples vessel-to-neuron communication-vasculo-neuronal coupling-a potential early event in cognitive decline.
Keywords: A1R; Adenosine; Hypoperfusion; Myogenic tone; Neurovascular unit.
Figures


References
-
- Fernandez-Seara MA, Mengual E, Vidorreta M, Aznarez-Sanado M, Loayza FR, Villagra F, et al. Cortical hypoperfusion in Parkinson’s disease assessed using arterial spin labeled perfusion MRI. Neuroimage. 2012;59(3):2743–2750. - PubMed
-
- de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer’s disease. J Alzheimers Dis. 2012;32(3):553–567. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

