Astrocyte regional heterogeneity revealed through machine learning-based glial neuroanatomical assays
- PMID: 33410136
- PMCID: PMC8113076
- DOI: 10.1002/cne.25105
Astrocyte regional heterogeneity revealed through machine learning-based glial neuroanatomical assays
Abstract
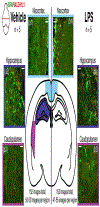
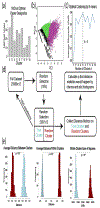
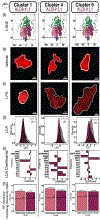
Evaluation of reactive astrogliosis by neuroanatomical assays represents a common experimental outcome for neuroanatomists. The literature demonstrates several conflicting results as to the accuracy of such measures. We posited that the diverging results within the neuroanatomy literature were due to suboptimal analytical workflows in addition to astrocyte regional heterogeneity. We therefore generated an automated segmentation workflow to extract features of glial fibrillary acidic protein (GFAP) and aldehyde dehydrogenase family 1, member L1 (ALDH1L1) labeled astrocytes with and without neuroinflammation. We achieved this by capturing multiplexed immunofluorescent confocal images of mouse brains treated with either vehicle or lipopolysaccharide (LPS) followed by implementation of our workflows. Using classical image analysis techniques focused on pixel intensity only, we were unable to identify differences between vehicle-treated and LPS-treated animals. However, when utilizing machine learning-based algorithms, we were able to (1) accurately predict which objects were derived from GFAP or ALDH1L1-stained images indicating that GFAP and ALDH1L1 highlight distinct morphological aspects of astrocytes, (2) we could predict which neuroanatomical region the segmented GFAP or ALDH1L1 object had been derived from, indicating that morphological features of astrocytes change as a function of neuroanatomical location. (3) We discovered a statistically significant, albeit not highly accurate, prediction of which objects had come from LPS versus vehicle-treated animals, indicating that although features exist capable of distinguishing LPS-treated versus vehicle-treated GFAP and ALDH1L1-segmented objects, that significant overlap between morphologies exists. We further determined that for most classification scenarios, nonlinear models were required for improved treatment class designations. We propose that unbiased automated image analysis techniques coupled with well-validated machine learning tools represent highly useful models capable of providing insights into neuroanatomical assays.
Keywords: astrocyte; clustering analysis; gliosis; machine learning; neuroanatomy; neuroinflammation.
© 2021 Wiley Periodicals LLC.
Figures







References
-
- Bergmeir C, & Benítez J (2012). Neural Networks in R Using the Stuttgart Neural Network Simulator: RSNNS. In (Vol. 46(7), pp. 1–26). Journal of Statistical Software.
-
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, … Barres BA (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci, 28(1), 264–278. doi: 10.1523/JNEUROSCI.4178-07.2008 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

