A phase 3, multicenter, randomized, double-blind, vehicle-controlled, parallel-group study of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis
- PMID: 33410265
- PMCID: PMC7986147
- DOI: 10.1111/1346-8138.15668
A phase 3, multicenter, randomized, double-blind, vehicle-controlled, parallel-group study of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis
Abstract
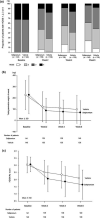
A phase 3 study was conducted to verify the efficacy and safety of 5% sofpironium bromide (BBI-4000) gel (hereinafter referred to as sofpironium) administrated for 6 weeks in Japanese patients with primary axillary hyperhidrosis. The primary efficacy end-point was the proportion of patients who satisfied both criteria of a Hyperhidrosis Disease Severity Score (HDSS) of 1 or 2 at the end of 6-week treatment and a 50% or more reduction in total gravimetric weight of sweat at the end of treatment relative to baseline. A total of 281 patients were randomized to receive 5% sofpironium (141 patients) or vehicle (140 patients), and all patients were included in the full analysis set (FAS). In the FAS, 70.1% of patients were female, and the median age was 35.0 years. The proportion of patients who achieved the primary efficacy end-point was 53.9% in the sofpironium group and 36.4% in the vehicle group, with a statistically significant difference of 17.5% (95% confidence interval, 6.02-28.93) between these two groups (P = 0.003). The incidence of adverse events was 44.0% in the sofpironium group and 30.7% in the vehicle group, and the incidence of adverse drug reactions was 16.3% in the sofpironium group and 5.0% in the vehicle group. Reported adverse events were generally mild or moderate in severity. In the sofpironium group, common events (incidence, ≥5%) were nasopharyngitis (14.2%) and dermatitis/erythema at the application site (8.5%/5.7%), with no serious adverse events reported. This study demonstrated the efficacy and safety of 5% sofpironium.
Keywords: BBI-4000; Hyperhidrosis Disease Severity Score; phase 3 study; primary axillary hyperhidrosis; sofpironium bromide gel.
© 2021 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
This study was funded by Kaken Pharmaceutical. H. Y. received a consultancy fee and/or commission fee from Kaken Pharmaceutical. H. Y., T. F., M. I., T. O., H. K., R. M., Y. M., A. K. and A. Y. received fees as resource speakers from Kaken Pharmaceutical. H. Y. received fees for arranging education from Kaken Pharmaceutical. O. T. has stock in Kaken Pharmaceutical. M. A., T. Y. and S. T. are employees of Kaken Pharmaceutical and have stock in Kaken Pharmaceutical. With funding from Kaken Pharmaceutical, Medical Professional Relations assisted in the writing and editing of this paper.
Figures

Similar articles
-
A phase III, 52-week, open-label study to evaluate the safety and efficacy of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis.J Dermatol. 2021 Aug;48(8):1149-1161. doi: 10.1111/1346-8138.15927. Epub 2021 May 26. J Dermatol. 2021. PMID: 34041788 Free PMC article. Clinical Trial.
-
Two-week prospective observational study of 5% sofpironium bromide gel in Japanese patients with primary axillary hyperhidrosis.J Dermatol. 2022 Jun;49(6):594-599. doi: 10.1111/1346-8138.16384. Epub 2022 Apr 8. J Dermatol. 2022. PMID: 35394087 Free PMC article.
-
Sofpironium topical gel, 12.45%, for the treatment of axillary hyperhidrosis: Pooled efficacy and safety results from 2 phase 3 randomized, controlled, double-blind studies.J Am Acad Dermatol. 2025 Jul;93(1):82-88. doi: 10.1016/j.jaad.2025.02.086. Epub 2025 Mar 5. J Am Acad Dermatol. 2025. PMID: 40054501 Clinical Trial.
-
Sofpironium Bromide: First Approval.Drugs. 2020 Dec;80(18):1981-1986. doi: 10.1007/s40265-020-01438-1. Drugs. 2020. PMID: 33236266 Review.
-
Efficacy and safety of topical glycopyrronium bromide in treating axillary hyperhidrosis: systematic review and meta-analysis.Sci Rep. 2024 Oct 19;14(1):24537. doi: 10.1038/s41598-024-74430-4. Sci Rep. 2024. PMID: 39424822 Free PMC article.
Cited by
-
A glycopyrronium bromide 1% cream for topical treatment of primary axillary hyperhidrosis: efficacy and safety results from a phase IIIa randomized controlled trial.Br J Dermatol. 2021 Aug;185(2):315-322. doi: 10.1111/bjd.19810. Epub 2021 Mar 2. Br J Dermatol. 2021. PMID: 33445205 Free PMC article. Clinical Trial.
-
Primary hyperhidrosis: an updated review.Drugs Context. 2025 Jun 16;14:2025-3-2. doi: 10.7573/dic.2025-3-2. eCollection 2025. Drugs Context. 2025. PMID: 40575073 Free PMC article. Review.
-
Bibliometric Analysis in Hyperhidrosis: Recent Publication Trends from 2015 to 2025.Skin Appendage Disord. 2025 May 8:1-16. doi: 10.1159/000545767. Online ahead of print. Skin Appendage Disord. 2025. PMID: 40510941 Free PMC article.
-
Efficacy of sofpironium bromide gel on clozapine-induced hypersalivation in patients with treatment-resistant schizophrenia: double-blind, controlled crossover study.BJPsych Open. 2023 Jan 13;9(1):e14. doi: 10.1192/bjo.2022.630. BJPsych Open. 2023. PMID: 36636808 Free PMC article.
-
A phase III, 52-week, open-label study to evaluate the safety and efficacy of 5% sofpironium bromide (BBI-4000) gel in Japanese patients with primary axillary hyperhidrosis.J Dermatol. 2021 Aug;48(8):1149-1161. doi: 10.1111/1346-8138.15927. Epub 2021 May 26. J Dermatol. 2021. PMID: 34041788 Free PMC article. Clinical Trial.
References
-
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Etiology and clinical work‐up. J Am Acad Dermatol 2019; 81: 657–666. - PubMed
-
- Fujimoto T, Kawahara K, Yokozeki H. Epidemiological study and considerations of focal hyperhidrosis in Japan. From questionnaire analysis. J Dermatol 2013; 40: 886–890. - PubMed
-
- Fujimoto T, Yokozeki H, Katayama I et al. Guidelines of the Japanese dermatological association, clinical guidelines for primary focal hyperhidrosis (revised edition in 2015). Jpn J Dermatol 2015; 125: 1379–1400. (article in Japanese).
-
- Ohshima Y, Tamada Y, Yokozeki H et al. The efficacy and safety of botulinum toxin type a in patients with primary axillary hyperhidrosis. Nishinihon. J Dermatology 2013; 75: 357–364. (article in Japanese).
-
- Nawrocki S, Cha J. The etiology, diagnosis, and management of hyperhidrosis: a comprehensive review: therapeutic options. J Am Acad Dermatol 2019; 81: 669–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

