Protein Mass-Modulated Effects in Alkaline Phosphatase
- PMID: 33410323
- PMCID: PMC8340299
- DOI: 10.1021/acs.biochem.0c00917
Protein Mass-Modulated Effects in Alkaline Phosphatase
Abstract
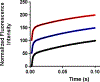
Recent experimental studies engaging isotopically substituted protein (heavy protein) have revealed that many, but not all, enzymatic systems exhibit altered chemical steps in response to an altered mass. The results have been interpreted as femtosecond protein dynamics at the active site being linked (or not) to transition-state barrier crossing. An altered enzyme mass can influence several kinetic parameters (kcat, Km, and kchem) in amounts of ≤30% relative to light enzymes. An early report on deuterium-labeled Escherichia coli alkaline phosphatase (AP) showed an unusually large enzyme kinetic isotope effect on kcat. We examined steady-state and chemical step properties of native AP, [2H]AP, and [2H,13C,15N]AP to characterize the role of heavy enzyme protein dynamics in reactions catalyzed by AP. Both [2H]- and [2H,13C,15N]APs showed unaltered steady-state and single-turnover rate constants. These findings characterize AP as one of the enzymes in which mass-dependent catalytic site dynamics is dominated by reactant-linked atomic motions. Two catalytic site zinc ions activate the oxygen nucleophiles in the catalytic site of AP. The mass of the zinc ions is unchanged in light and heavy APs. They are essentially linked to catalysis and provide a possible explanation for the loss of linkage between catalysis and protein mass in these enzymes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
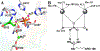
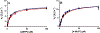
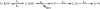
Similar articles
-
Differential catalytic promiscuity of the alkaline phosphatase superfamily bimetallo core reveals mechanistic features underlying enzyme evolution.J Biol Chem. 2017 Dec 22;292(51):20960-20974. doi: 10.1074/jbc.M117.788240. Epub 2017 Oct 25. J Biol Chem. 2017. PMID: 29070681 Free PMC article.
-
Kinetic isotope effects for alkaline phosphatase reactions: implications for the role of active-site metal ions in catalysis.J Am Chem Soc. 2007 Aug 8;129(31):9789-98. doi: 10.1021/ja072196+. Epub 2007 Jul 14. J Am Chem Soc. 2007. PMID: 17630738 Free PMC article.
-
Coordination sphere of the third metal site is essential to the activity and metal selectivity of alkaline phosphatases.Protein Sci. 2010 Jan;19(1):75-84. doi: 10.1002/pro.284. Protein Sci. 2010. PMID: 19916164 Free PMC article.
-
The mechanism of the alkaline phosphatase reaction: insights from NMR, crystallography and site-specific mutagenesis.FEBS Lett. 1999 Nov 26;462(1-2):7-11. doi: 10.1016/s0014-5793(99)01448-9. FEBS Lett. 1999. PMID: 10580082 Review.
-
Structure and mechanism of alkaline phosphatase.Annu Rev Biophys Biomol Struct. 1992;21:441-83. doi: 10.1146/annurev.bb.21.060192.002301. Annu Rev Biophys Biomol Struct. 1992. PMID: 1525473 Review.
Cited by
-
Allosteric activation unveils protein-mass modulation of ATP phosphoribosyltransferase product release.Commun Chem. 2024 Apr 6;7(1):77. doi: 10.1038/s42004-024-01165-8. Commun Chem. 2024. PMID: 38582930 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

