Synthesis and Biological Evaluation of (2 S,2' S)-Lomaiviticin A
- PMID: 33410680
- PMCID: PMC8174553
- DOI: 10.1021/jacs.0c11960
Synthesis and Biological Evaluation of (2 S,2' S)-Lomaiviticin A
Abstract
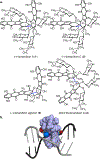
(-)-Lomaiviticin A (1) is a genotoxic C2-symmetric metabolite that arises from the formal dimerization of two bis(glycosylated) diazotetrahydrobenzo[b]fluorenes. Here we present a synthesis of the monomer 17 and its coupling to form (2S,2'S)-lomaiviticin A (4), an unnatural diastereomer of 1. (2S,2'S)-Lomaiviticin A (4) is significantly less genotoxic, a result we attribute to changes in the orientation of the diazofluorene and carbohydrate residues, relative to 1. These data bring the importance of the configuration of the conjoining bond to light and place the total synthesis of 1 itself within reach.
Figures





References
-
- Isolation of (–)-lomaiviticin A (1):
- He H; Ding WD; Bernan VS; Richardson AD; Ireland CM; Greenstein M; Ellestad GA; Carter GT Lomaiviticins A and B, Potent Antitumor Antibiotics from Micromonospora lomaivitiensis. J. Am. Chem. Soc 2001, 123, 5362. For elucidation of the absolute stereochemistry of 1, see: - PubMed
- Woo CM; Beizer NE; Janso JE; Herzon SB Isolation of Lomaiviticins C–E. Transformation of Lomaiviticin C to Lomaiviticin A, Complete Structure Elucidation of Lomaiviticin A, and Structure–Activity Analyses. J. Am. Chem. Soc 2012, 134, 15285. - PubMed
-
- For reviews, see:
- Gould SJ. Biosynthesis of the Kinamycins. Chem. Rev 1997, 97, 2499. - PubMed
- Marco-Contelles J; Molina MT Naturally Occurring Diazo Compounds: The Kinamycins. Curr. Org. Chem 2003, 7, 1433.
- Arya DP. Diazo and Diazonium DNA Cleavage Agents: Studies on Model Systems and Natural Product Mechanisms of Action. Top. Heterocycl. Chem 2006, 2, 129.
- Nawrat CC; Moody CJ Natural Products Containing a Diazo Group. Nat. Prod. Rep 2011, 28, 1426. - PubMed
- Herzon SB. The Kinamycins. In Total Synthesis of Natural Products. At the Frontiers of Organic Chemistry; Li JJ, Corey EJ, Eds.; Springer-Verlag: Berlin Heidelberg, 2012; pp 39.
- Herzon SB; Woo CM The Diazofluorene Antitumor Antibiotics: Structural Elucidation, Biosynthetic, Synthetic, and Chemical Biological Studies. Nat. Prod. Rep 2012, 29, 87. - PubMed
- Herzon SB The Mechanism of Action of (–)-Lomaiviticin A. Acc. Chem. Res 2017, 50, 2577. - PMC - PubMed
-
- Mulcahy SP; Woo CM; Ding WD; Ellestad GA; Herzon SB Characterization of a Reductively-Activated Elimination Pathway Relevant to the Biological Chemistry of the Kinamycins and Lomaiviticins. Chem. Sci 2012, 3, 1070.
- Colis LC; Woo CM; Hegan DC; Li Z; Glazer PM; Herzon SB The Cytotoxicity of (–)-Lomaiviticin A Arises from Induction of Double-strand Breaks in DNA. Nat. Chem 2014, 6, 504. - PMC - PubMed
- Woo CM; Ranjan N; Arya DP; Herzon SB Analysis of Diazofluorene DNA Binding and Damaging Activity: DNA Cleavage by a Synthetic Monomeric Diazofluorene. Angew. Chem., Int. Ed 2014, 53, 9325. - PMC - PubMed
- Colis LC; Hegan DC; Kaneko M; Glazer PM; Herzon SB Mechanism of Action Studies of Lomaiviticin A and the Monomeric Lomaiviticin Aglycon. Selective and Potent Activity Toward DNA Double-strand Break Repair-deficient Cell Lines. J. Am. Chem. Soc 2015, 137, 5741. - PMC - PubMed
- Woo CM; Li Z; Paulson EK; Herzon SB Structural Basis for DNA Cleavage by the Potent Antiproliferative Agent (–)-Lomaiviticin A. Proc. Natl. Acad. Sci. U. S. A 2016, 113, 2851. - PMC - PubMed
- Colis LC; Herzon SB Synergistic Potentiation of (−)-Lomaiviticin A Cytotoxicity by the ATR Inhibitor VE-821. Bioorg. Med. Chem. Lett 2016, 26, 3122. - PMC - PubMed
- Xue M; Herzon SB Mechanism of Nucleophilic Activation of (−)-Lomaiviticin A. J. Am. Chem. Soc 2016, 138, 15559. - PMC - PubMed
-
- For the first proposal that the diazo residue of (–)-lomaiviticin A (1) may be electrophilic, see:
- Laufer RS; Dmitrienko GI Diazo Group Electrophilicity in Kinamycins and Lomaiviticin A: Potential Insights into the Molecular Mechanism of Antibacterial and Antitumor Activity. J. Am. Chem. Soc 2002, 124, 1854. For the first proposal that vinyl radicals form from 1, see: - PubMed
- Feldman KS; Eastman KJ A Proposal for the Mechanism-of-Action of Diazoparaquinone Natural Products. J. Am. Chem. Soc 2005, 127, 15344. - PMC - PubMed
- Feldman KS; Eastman KJ Studies on the Mechanism of Action of Prekinamycin, a Member of the Diazoparaquinone Family of Natural Products: Evidence for Both sp2 Radical and Orthoquinonemethide Intermediates. J. Am. Chem. Soc 2006, 128, 12562. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

