Efficacy of Osimertinib Plus Bevacizumab vs Osimertinib in Patients With EGFR T790M-Mutated Non-Small Cell Lung Cancer Previously Treated With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: West Japan Oncology Group 8715L Phase 2 Randomized Clinical Trial
- PMID: 33410885
- PMCID: PMC7791398
- DOI: 10.1001/jamaoncol.2020.6758
Efficacy of Osimertinib Plus Bevacizumab vs Osimertinib in Patients With EGFR T790M-Mutated Non-Small Cell Lung Cancer Previously Treated With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: West Japan Oncology Group 8715L Phase 2 Randomized Clinical Trial
Abstract
Importance: Although treatment with first-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) plus antiangiogenic inhibitor has shown promising efficacies in patients with EGFR-mutated lung adenocarcinoma, recent single-arm studies have suggested that osimertinib plus antiangiogenic inhibitor might not work synergistically.
Objective: To explore the efficacy and safety of osimertinib plus bevacizumab compared with osimertinib alone in patients with lung adenocarcinoma with EGFR T790M mutation.
Design, setting, and participants: Patients with advanced lung adenocarcinoma that progressed with prior EGFR-TKI treatment (other than third-generation TKI) and acquired EGFR T790M mutation were enrolled. This study comprises a lead-in part with 6 patients and a subsequent phase 2 part. In phase 2, patients were randomized to osimertinib plus bevacizumab or osimertinib alone in a 1:1 ratio.
Interventions: The combination arm received oral osimertinib (80 mg, every day) plus intravenous bevacizumab (15 mg/kg, every 3 weeks) until progression or unacceptable toxic effects. The control arm received osimertinib monotherapy.
Main outcomes and measures: The primary end point was progression-free survival (PFS) assessed by investigators. Secondary end points consisted of overall response rate, time to treatment failure, overall survival, and safety.
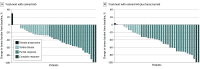
Results: From August 2017 through September 2018, a total of 87 patients were registered (6 in the lead-in part and 81 in the phase 2 part [intention-to-treat population]). Among those randomized, the median (range) age was 68 (41-82) years; 33 (41%) were male; 37 (46%) had an Eastern Cooperative Oncology Group performance status of 0; and 21 (26%) had brain metastasis. Although the overall response rate was better with osimertinib plus bevacizumab than osimertinib alone (68% vs 54%), median PFS was not longer with osimertinib plus bevacizumab (9.4 months vs 13.5 months; adjusted hazard ratio, 1.44; 80% CI, 1.00 to 2.08; P = .20). Median time to treatment failure was also shorter in the combination arm vs the osimertinib arm (8.4 months vs 11.2 months; P = .12). Median overall survival was not different in the combination arm vs osimertinib arm (not reached vs 22.1 months; P = .96). In the combination arm, common adverse events of grade 3 or higher were proteinuria (n = 9; 23%), hypertension (n = 8; 20%).
Conclusions and relevance: In this randomized clinical trial comparing osimertinib plus bevacizumab vs osimertinib alone, the combination arm failed to show prolongation of PFS in patients with advanced lung adenocarcinoma with EGFR T790M mutation.
Trial registration: UMIN Clinical Trials Registry Identifier: UMIN000023761.
Conflict of interest statement
Figures


Comment in
-
When the Signal From Phase 2 Research Should Be a Warning Sign.JAMA Oncol. 2021 Mar 1;7(3):394. doi: 10.1001/jamaoncol.2020.6598. JAMA Oncol. 2021. PMID: 33410909 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

