Letter to editor
- PMID: 33411325
- PMCID: PMC7788163
- DOI: 10.1007/s10815-020-02024-w
Letter to editor
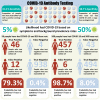
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

