Evaluation of the Structure-Activity Relationship of Microtubule-Targeting 1,2,4-Triazolo[1,5- a]pyrimidines Identifies New Candidates for Neurodegenerative Tauopathies
- PMID: 33411523
- PMCID: PMC8569887
- DOI: 10.1021/acs.jmedchem.0c01605
Evaluation of the Structure-Activity Relationship of Microtubule-Targeting 1,2,4-Triazolo[1,5- a]pyrimidines Identifies New Candidates for Neurodegenerative Tauopathies
Abstract
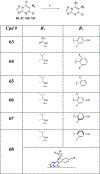
Studies in tau and Aβ plaque transgenic mouse models demonstrated that brain-penetrant microtubule (MT)-stabilizing compounds, including the 1,2,4-triazolo[1,5-a]pyrimidines, hold promise as candidate treatments for Alzheimer's disease and related neurodegenerative tauopathies. Triazolopyrimidines have already been investigated as anticancer agents; however, the antimitotic activity of these compounds does not always correlate with stabilization of MTs in cells. Indeed, previous studies from our laboratories identified a critical role for the fragment linked at C6 in determining whether triazolopyrimidines promote MT stabilization or, conversely, disrupt MT integrity in cells. To further elucidate the structure-activity relationship (SAR) and to identify potentially improved MT-stabilizing candidates for neurodegenerative disease, a comprehensive set of 68 triazolopyrimidine congeners bearing structural modifications at C6 and/or C7 was designed, synthesized, and evaluated. These studies expand upon prior understanding of triazolopyrimidine SAR and enabled the identification of novel analogues that, relative to the existing lead, exhibit improved physicochemical properties, MT-stabilizing activity, and pharmacokinetics.
Figures







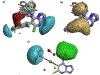




Similar articles
-
Characterization of Brain-Penetrant Pyrimidine-Containing Molecules with Differential Microtubule-Stabilizing Activities Developed as Potential Therapeutic Agents for Alzheimer's Disease and Related Tauopathies.J Pharmacol Exp Ther. 2016 May;357(2):432-50. doi: 10.1124/jpet.115.231175. Epub 2016 Mar 15. J Pharmacol Exp Ther. 2016. PMID: 26980057 Free PMC article.
-
A brain-penetrant triazolopyrimidine enhances microtubule-stability, reduces axonal dysfunction and decreases tau pathology in a mouse tauopathy model.Mol Neurodegener. 2018 Nov 7;13(1):59. doi: 10.1186/s13024-018-0291-3. Mol Neurodegener. 2018. PMID: 30404654 Free PMC article.
-
Design, synthesis and evaluation of photoactivatable derivatives of microtubule (MT)-active [1,2,4]triazolo[1,5-a]pyrimidines.Bioorg Med Chem Lett. 2018 Jul 1;28(12):2180-2183. doi: 10.1016/j.bmcl.2018.05.010. Epub 2018 May 5. Bioorg Med Chem Lett. 2018. PMID: 29764743 Free PMC article.
-
Brain-penetrant microtubule-stabilizing compounds as potential therapeutic agents for tauopathies.Biochem Soc Trans. 2012 Aug;40(4):661-6. doi: 10.1042/BST20120010. Biochem Soc Trans. 2012. PMID: 22817712 Free PMC article. Review.
-
Microtubule stabilizing agents as potential treatment for Alzheimer's disease and related neurodegenerative tauopathies.J Med Chem. 2012 Nov 8;55(21):8979-96. doi: 10.1021/jm301079z. Epub 2012 Sep 28. J Med Chem. 2012. PMID: 23020671 Free PMC article. Review.
Cited by
-
Structure-Activity Relationships, Tolerability and Efficacy of Microtubule-Active 1,2,4-Triazolo[1,5-a]pyrimidines as Potential Candidates to Treat Human African Trypanosomiasis.ChemMedChem. 2023 Oct 17;18(20):e202300193. doi: 10.1002/cmdc.202300193. Epub 2023 Jul 24. ChemMedChem. 2023. PMID: 37429821 Free PMC article.
-
Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy.Pharmaceuticals (Basel). 2024 Dec 28;18(1):28. doi: 10.3390/ph18010028. Pharmaceuticals (Basel). 2024. PMID: 39861091 Free PMC article. Review.
-
Design, Synthesis, and Evaluation of An Anti-trypanosomal [1,2,4]Triazolo[1,5-a]pyrimidine Probe for Photoaffinity Labeling Studies.ChemMedChem. 2024 Apr 16;19(8):e202300656. doi: 10.1002/cmdc.202300656. Epub 2024 Feb 12. ChemMedChem. 2024. PMID: 38277231 Free PMC article.
-
Synthesis of Ethyl Pyrimidine-Quinolincarboxylates Selected from Virtual Screening as Enhanced Lactate Dehydrogenase (LDH) Inhibitors.Int J Mol Sci. 2024 Sep 9;25(17):9744. doi: 10.3390/ijms25179744. Int J Mol Sci. 2024. PMID: 39273691 Free PMC article.
-
Alzheimer's disease: an axonal injury disease?Front Aging Neurosci. 2023 Oct 19;15:1264448. doi: 10.3389/fnagi.2023.1264448. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37927337 Free PMC article. Review.
References
-
- Lee VMY; Goedert M; Trojanowski JQ Neurodegenerative tauopathies. Annu. Rev. Neurosci 2001, 24, 1121–1159. - PubMed
-
- Ballatore C; Lee VMY; Trojanowski JQ Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci 2007, 8, 663–672. - PubMed
-
- Sadleir KR; Kandalepas PC; Buggia-Prevot V; Nicholson DA; Thinakaran G; Vassar R Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Abeta generation in Alzheimer’s disease. Acta neuropathol. 2016, 132, 235–256. - PMC - PubMed
-
- Lee VMY; Daughenbaugh R; Trojanowski JQ Microtubule stabilizing drugs for the treatment of Alzheimers-disease. Neurobiol. Aging 1994, 15, S87–S89. - PubMed
-
- Brunden KR; Zhang B; Carroll J; Yao Y; Potuzak JS; Hogan AM; Iba M; James MJ; Xie SX; Ballatore C; Smith AB III; Lee VM; Trojanowski JQ Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci 2010, 30, 13861–13866. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

