Extracellular vesicles released from the filarial parasite Brugia malayi downregulate the host mTOR pathway
- PMID: 33411714
- PMCID: PMC7790274
- DOI: 10.1371/journal.pntd.0008884
Extracellular vesicles released from the filarial parasite Brugia malayi downregulate the host mTOR pathway
Abstract
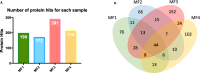
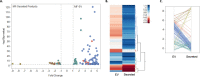
We have previously shown that the microfilarial (mf) stage of Brugia malayi can inhibit the mammalian target of rapamycin (mTOR; a conserved serine/threonine kinase critical for immune regulation and cellular growth) in human dendritic cells (DC) and we have proposed that this mTOR inhibition is associated with the DC dysfunction seen in filarial infections. Extracellular vesicles (EVs) contain many proteins and nucleic acids including microRNAs (miRNAs) that might affect a variety of intracellular pathways. Thus, EVs secreted from mf may elucidate the mechanism by which the parasite is able to modulate the host immune response during infection. EVs, purified from mf of Brugia malayi and confirmed by size through nanoparticle tracking analysis, were assessed by miRNA microarrays (accession number GSE157226) and shown to be enriched (>2-fold, p-value<0.05, FDR = 0.05) for miR100, miR71, miR34, and miR7. The microarray analysis compared mf-derived EVs and mf supernatant. After confirming their presence in EVs using qPCR for these miRNA targets, web-based target predictions (using MIRPathv3, TarBAse and MicroT-CD) predicted that miR100 targeted mTOR and its downstream regulatory protein 4E-BP1. Our previous data with live parasites demonstrated that mf downregulate the phosphorylation of mTOR and its downstream effectors. Additionally, our proteomic analysis of the mf-derived EVs revealed the presence of proteins commonly found in these vesicles (data are available via ProteomeXchange with identifier PXD021844). We confirmed internalization of mf-derived EVs by human DCs and monocytes using confocal microscopy and flow cytometry, and further demonstrated through flow cytometry, that mf-derived EVs downregulate the phosphorylation of mTOR in human monocytes (THP-1 cells) to the same degree that rapamycin (a known mTOR inhibitor) does. Our data collectively suggest that mf release EVs that interact with host cells, such as DC, to modulate host responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Microfilariae of Brugia malayi Inhibit the mTOR Pathway and Induce Autophagy in Human Dendritic Cells.Infect Immun. 2016 Aug 19;84(9):2463-72. doi: 10.1128/IAI.00174-16. Print 2016 Sep. Infect Immun. 2016. PMID: 27297394 Free PMC article.
-
Brugia malayi filarial helminth-derived extracellular vesicles suppress antigen presenting cell function and antigen-specific CD4+ T cell responses.Front Immunol. 2024 Oct 7;15:1436818. doi: 10.3389/fimmu.2024.1436818. eCollection 2024. Front Immunol. 2024. PMID: 39434874 Free PMC article.
-
Human Leukocytes Kill Brugia malayi Microfilariae Independently of DNA-Based Extracellular Trap Release.PLoS Negl Trop Dis. 2017 Jan 3;11(1):e0005279. doi: 10.1371/journal.pntd.0005279. eCollection 2017 Jan. PLoS Negl Trop Dis. 2017. PMID: 28045905 Free PMC article.
-
Immune evasion genes from filarial nematodes.Int J Parasitol. 2001 Jul;31(9):889-98. doi: 10.1016/s0020-7519(01)00213-2. Int J Parasitol. 2001. PMID: 11406138 Review.
-
Emerging roles for extracellular vesicles in Schistosoma infection.Acta Trop. 2022 Aug;232:106467. doi: 10.1016/j.actatropica.2022.106467. Epub 2022 Apr 12. Acta Trop. 2022. PMID: 35427535 Review.
Cited by
-
Lack of detectable short-term effects of a single dose of ivermectin on the human immune system.Parasit Vectors. 2021 Jun 5;14(1):304. doi: 10.1186/s13071-021-04810-6. Parasit Vectors. 2021. PMID: 34090504 Free PMC article. Clinical Trial.
-
Unraveling the microRNAs Involved in Fasciolosis: Master Regulators of the Host-Parasite Crosstalk.Int J Mol Sci. 2024 Dec 29;26(1):204. doi: 10.3390/ijms26010204. Int J Mol Sci. 2024. PMID: 39796061 Free PMC article. Review.
-
Emerging Roles of Micrornas in Veterinary Cardiology.Vet Sci. 2022 Sep 28;9(10):533. doi: 10.3390/vetsci9100533. Vet Sci. 2022. PMID: 36288146 Free PMC article. Review.
-
Identification of biomarker candidates for filarial parasite infections by analysis of extracellular vesicles.Front Parasitol. 2023 Oct 23;2:1281092. doi: 10.3389/fpara.2023.1281092. eCollection 2023. Front Parasitol. 2023. PMID: 39816829 Free PMC article.
-
Exploring extracellular vesicles in zoonotic helminth biology: implications for diagnosis, therapeutic and delivery.Front Cell Infect Microbiol. 2024 Aug 6;14:1424838. doi: 10.3389/fcimb.2024.1424838. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39165921 Free PMC article. Review.
References
-
- Global programme to eliminate lymphatic filariasis: progress report, 2011. Weekly Epidemiological Record. 2012. September; 87(37): 346–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

