CX3CR1 is a prerequisite for the development of cardiac hypertrophy and left ventricular dysfunction in mice upon transverse aortic constriction
- PMID: 33411754
- PMCID: PMC7790399
- DOI: 10.1371/journal.pone.0243788
CX3CR1 is a prerequisite for the development of cardiac hypertrophy and left ventricular dysfunction in mice upon transverse aortic constriction
Abstract
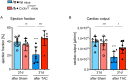
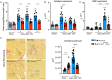
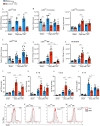
The CX3CL1/CX3CR1 axis mediates recruitment and extravasation of CX3CR1-expressing subsets of leukocytes and plays a pivotal role in the inflammation-driven pathology of cardiovascular disease. The cardiac immune response differs depending on the underlying causes. This suggests that for the development of successful immunomodulatory therapy in heart failure due to chronic pressure overload induced left ventricular (LV) hypertrophy, the underlying immune patterns must be examined. Here, the authors demonstrate that Fraktalkine-receptor CX3CR1 is a prerequisite for the development of cardiac hypertrophy and left ventricular dysfunction in a mouse model of transverse aortic constriction (TAC). The comparison of C57BL/6 mice with CX3CR1 deficient mice displayed reduced LV hypertrophy and preserved cardiac function in response to pressure overload in mice lacking CX3CR1. Moreover, the normal immune response following TAC induced pressure overload which is dominated by Ly6Clow macrophages changed to an early pro-inflammatory immune response driven by neutrophils, Ly6Chigh macrophages and altered cytokine expression pattern in CX3CR1 deficient mice. In this early inflammatory phase of LV hypertrophy Ly6Chigh monocytes infiltrated the heart in response to a C-C chemokine ligand 2 burst. CX3CR1 expression impacts the immune response in the development of LV hypertrophy and its absence has clear cardioprotective effects. Hence, suppression of CX3CR1 may be an important immunomodulatory therapeutic target to ameliorate pressure-overload induced heart failure.
Conflict of interest statement
This study was supported by the Flow-cytometry Core Facility and by the House for Experimental Therapy (HET) of the medical faculty of Bonn University. C. K. W. and L. E. were supported by the Else Kröner-Forschungskolleg Bonn and BONFOR in Bonn; C. K. W., S. Z. and C. K. are funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Grant No. 397484323 – Project number 426093965. C. K. and S.Z. are members of the excellence cluster ‘‘ImmunoSensation’’ at Bonn University. There are no patents, products in development or marketed products to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Velten M, Duerr GD, Pessies T, Schild J, Lohner R, Mersmann J, et al. Priming with synthetic oligonucleotides attenuates pressure overload-induced inflammation and cardiac hypertrophy in mice. Cardiovasc Res. 2012;96(3):422–32. Epub 2012/09/15. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

