Looking into the future: Gene and cell therapies for glaucoma
- PMID: 33411993
- PMCID: PMC7979454
- DOI: 10.1111/vop.12858
Looking into the future: Gene and cell therapies for glaucoma
Abstract
Glaucoma is a complex group of optic neuropathies that affects both humans and animals. Intraocular pressure (IOP) elevation is a major risk factor that results in the loss of retinal ganglion cells (RGCs) and their axons. Currently, lowering IOP by medical and surgical methods is the only approved treatment for primary glaucoma, but there is no cure, and vision loss often progresses despite therapy. Recent technologic advances provide us with a better understanding of disease mechanisms and risk factors; this will permit earlier diagnosis of glaucoma and initiation of therapy sooner and more effectively. Gene and cell therapies are well suited to target these mechanisms specifically with the potential to achieve a lasting therapeutic effect. Much progress has been made in laboratory settings to develop these novel therapies for the eye. Gene and cell therapies have already been translated into clinical application for some inherited retinal dystrophies and age-related macular degeneration (AMD). Except for the intravitreal application of ciliary neurotrophic factor (CNTF) by encapsulated cell technology for RGC neuroprotection, there has been no other clinical translation of gene and cell therapies for glaucoma so far. Possible application of gene and cell therapies consists of long-term IOP control via increased aqueous humor drainage, including inhibition of fibrosis following filtration surgery, RGC neuroprotection and neuroregeneration, modification of ocular biomechanics for improved IOP tolerance, and inhibition of inflammation and neovascularization to prevent the development of some forms of secondary glaucoma.
Keywords: cell therapy; gene therapy; glaucoma; intraocular pressure (IOP); neuroprotection; ocular biomechanics.
© 2021 American College of Veterinary Ophthalmologists.
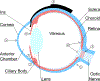
Figures


References
-
- Gelatt KN, MacKay EO. Prevalence of the breed-related glaucomas in pure-bred dogs in North America. Veterinary ophthalmology. 2004;7(2):97–111. - PubMed
-
- Gelatt KN, MacKay EO. Secondary glaucomas in the dog in North America. Veterinary ophthalmology. 2004;7(4):245–259. - PubMed
-
- Strom AR, Hassig M, Iburg TM, Spiess BM. Epidemiology of canine glaucoma presented to University of Zurich from 1995 to 2009. Part 2: secondary glaucoma (217 cases). Veterinary ophthalmology. 2011;14(2):127–132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

