Tractography-Pathology Correlations in Traumatic Brain Injury: A TRACK-TBI Study
- PMID: 33412995
- PMCID: PMC8165468
- DOI: 10.1089/neu.2020.7373
Tractography-Pathology Correlations in Traumatic Brain Injury: A TRACK-TBI Study
Abstract
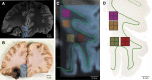
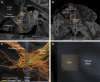
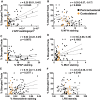
Diffusion tractography magnetic resonance imaging (MRI) can infer changes in network connectivity in patients with traumatic brain injury (TBI), but the pathological substrates of disconnected tracts have not been well defined because of a lack of high-resolution imaging with histopathological validation. We developed an ex vivo MRI protocol to analyze tract terminations at 750-μm isotropic resolution, followed by histopathological evaluation of white matter pathology, and applied these methods to a 60-year-old man who died 26 days after TBI. Analysis of 74 cerebral hemispheric white matter regions revealed a heterogeneous distribution of tract disruptions. Associated histopathology identified variable white matter injury with patchy deposition of amyloid precursor protein (APP), loss of neurofilament-positive axonal processes, myelin dissolution, astrogliosis, microgliosis, and perivascular hemosiderin-laden macrophages. Multiple linear regression revealed that tract disruption strongly correlated with the density of APP-positive axonal swellings and neurofilament loss. Ex vivo diffusion MRI can detect tract disruptions in the human brain that reflect axonal injury.
Keywords: MRI; contusion; neuropathology; tractography; traumatic axonal injury; traumatic brain injury.
Conflict of interest statement
Dr. Fischl has a financial interest in CorticoMetrics, a company whose medical pursuits focus on brain imaging and measurement technologies. His interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
The United States Department of Energy supports Dr. Manley for a precision medicine collaboration. One Mind has provided funding for TRACK-TBI patient stipends and support to clinical sites. Dr. Manley has received an unrestricted gift from the NFL to the UCSF Foundation to support research efforts of the TRACK-TBI NETWORK. Dr. Manley has also received funding from NeuroTrauma Sciences LLC to support TRACK-TBI data curation efforts. Additionally, Abbott Laboratories has provided funding for add-in TRACK-TBI clinical studies.
Ms. Markowitz receives salary support from the United States Department of Energy precision medicine collaboration and One Mind.
Figures


References
-
- Newcombe, V.F., Williams, G.B., Scoffings, D., Cross, J., Carpenter, T.A., Pickard, J.D., and Menon, D.K. (2010). Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. J. Neurol. Neurosurg. Psychiatry 81, 552–561 - PubMed
-
- Fernandez-Espejo, D., Soddu, A., Cruse, D., Palacios, E.M., Junque, C., Vanhaudenhuyse, A., Rivas, E., Newcombe, V., Menon, D.K., Pickard, J.D., Laureys, S., and Owen, A.M. (2012). A role for the default mode network in the bases of disorders of consciousness. Ann. Neurol. 72, 335–343 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

