Methodology in core outcome set (COS) development: the impact of patient interviews and using a 5-point versus a 9-point Delphi rating scale on core outcome selection in a COS development study
- PMID: 33413129
- PMCID: PMC7791855
- DOI: 10.1186/s12874-020-01197-3
Methodology in core outcome set (COS) development: the impact of patient interviews and using a 5-point versus a 9-point Delphi rating scale on core outcome selection in a COS development study
Abstract
Background: As the development of core outcome sets (COS) increases, guidance for developing and reporting high-quality COS continues to evolve; however, a number of methodological uncertainties still remain. The objectives of this study were: (1) to explore the impact of including patient interviews in developing a COS, (2) to examine the impact of using a 5-point versus a 9-point rating scale during Delphi consensus methods on outcome selection and (3) to inform and contribute to COS development methodology by advancing the evidence base on COS development techniques.
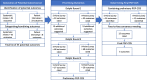
Methods: Semi-structured patient interviews and a nested randomised controlled parallel group trial as part of the Pelvic Girdle Pain Core Outcome Set project (PGP-COS). Patient interviews, as an adjunct to a systematic review of outcomes reported in previous studies, were undertaken to identify preliminary outcomes for including in a Delphi consensus survey. In the Delphi survey, participants were randomised (1:1) to a 5-point or 9-point rating scale for rating the importance of the list of preliminary outcomes.
Results: Four of the eight patient interview derived outcomes were included in the preliminary COS, however, none of these outcomes were included in the final PGP-COS. The 5-point rating scale resulted in twice as many outcomes reaching consensus after the 3-round Delphi survey compared to the 9-point scale. Consensus on all five outcomes included in the final PGP-COS was achieved by participants allocated the 5-point rating scale, whereas consensus on four of these was achieved by those using the 9-point scale.
Conclusions: Using patient interviews to identify preliminary outcomes as an adjunct to conducting a systematic review of outcomes measured in the literature did not appear to influence outcome selection in developing the COS in this study. The use of different rating scales in a Delphi survey, however, did appear to impact on outcome selection. The 5-point scale demonstrated greater congruency than the 9-point scale with the outcomes included in the final PGP-COS. Future research to substantiate our findings and to explore the impact of other rating scales on outcome selection during COS development, however, is warranted.
Keywords: Consensus methods; Core outcome set; Delphi methods; Patient interviews; Pelvic girdle pain; Rating scales.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous