Immune checkpoint inhibitors-related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems
- PMID: 33413213
- PMCID: PMC7791701
- DOI: 10.1186/s12885-020-07741-0
Immune checkpoint inhibitors-related myocarditis in patients with cancer: an analysis of international spontaneous reporting systems
Abstract
Background: Immune checkpoint inhibitors-induced myocarditis presents unique clinical challenges. Here, we assessed post-marketing safety of cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), programmed cell death-1 (PD-1), and programmed death-ligand 1 (PD-L1) inhibitors by mining the real-world data reported in two international pharmacovigilance databases.
Methods: We analyzed immune checkpoint inhibitors (ICIs)-associated fatal adverse drug events (ADEs) reports from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) collected from July 1, 2014 to December 31, 2019 and data from EudraVigilance (EV) database accessed on February 29, 2020. Three different data mining approaches were used to detect the signal of fatal myocarditis caused by ICIs.
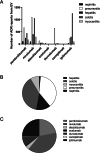
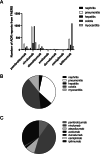
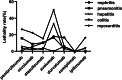
Results: Based on 7613 ICIs-related ADEs reported to the EV database and 5786 ICIs-associated ADEs submitted to the FAERS database, the most frequently reported ADE was ipilimumab-related colitis. For myocarditis, nivolumab-associated myocarditis was the most common. Among the five fatal toxic effects associated with ICIs, the lethality rate of myocarditis was the highest. Therefore, we further analyzed ICI-associated myocarditis and found that elderly patients and male patients were more likely to develop ICIs-related myocarditis. The results of signal detection showed that the risk signal of avelumab-related myocarditis detected by reporting odds ratio (ROR) method and proportional reporting ratios (PRR) method was the highest, whereas the signal strength of ipilimumab-related myocarditis detected by Bayesian confidence propagation neural networks (BCPNN) method was the strongest.
Conclusion: The findings of this study indicated the potential safety issues of developing myocarditis when using ICIs, which were consistent with the results of previous clinical trials and could provide a reference for clinical workers when using ICIs.
Keywords: Adverse drug reactions; Immune checkpoint inhibitors; Myocarditis; Signal detection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Immune checkpoint inhibitor-associated myocarditis and pericarditis: a pharmacovigilance study based on the FAERS database.BMC Cancer. 2025 Aug 9;25(1):1294. doi: 10.1186/s12885-025-14668-x. BMC Cancer. 2025. PMID: 40783691 Free PMC article.
-
Colitis following the use of immune checkpoint inhibitors: A real-world analysis of spontaneous reports submitted to the FDA adverse event reporting system.Int Immunopharmacol. 2020 Jul;84:106601. doi: 10.1016/j.intimp.2020.106601. Epub 2020 May 16. Int Immunopharmacol. 2020. PMID: 32422528
-
Thromboembolic events associated with immune checkpoint inhibitors: A real-world study of data from the food and drug administration adverse event reporting system (FAERS) database.Int Immunopharmacol. 2021 Sep;98:107818. doi: 10.1016/j.intimp.2021.107818. Epub 2021 Jun 12. Int Immunopharmacol. 2021. PMID: 34130149
-
Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis.JAMA Oncol. 2018 Dec 1;4(12):1721-1728. doi: 10.1001/jamaoncol.2018.3923. JAMA Oncol. 2018. PMID: 30242316 Free PMC article.
-
PD-1/PD-L1 inhibitor-induced immune thrombocytopenia: A pharmacovigilance study and systematic review.Int Immunopharmacol. 2024 Mar 10;129:111606. doi: 10.1016/j.intimp.2024.111606. Epub 2024 Feb 14. Int Immunopharmacol. 2024. PMID: 38359661
Cited by
-
Pharmacovigilance study on the reporting frequency of atrial fibrillation with immune checkpoint inhibitors: insights from FDA Adverse Event Reporting System.Ther Adv Drug Saf. 2025 Apr 21;16:20420986241312497. doi: 10.1177/20420986241312497. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40290514 Free PMC article.
-
Risk Factors for Immune Checkpoint Inhibitor-Related Myocarditis: An Integrative Review.J Adv Pract Oncol. 2024 Mar;15(2):111-123. doi: 10.6004/jadpro.2024.15.2.4. Epub 2024 Mar 1. J Adv Pract Oncol. 2024. PMID: 39132554 Free PMC article. Review.
-
Intrinsic Differences in Immune Checkpoint Inhibitor-Induced Myocarditis: A Retrospective Analysis of Real World Data.Front Pharmacol. 2022 Jul 5;13:914928. doi: 10.3389/fphar.2022.914928. eCollection 2022. Front Pharmacol. 2022. PMID: 35865949 Free PMC article.
-
Rheumatic Manifestations and Diseases From Immune Checkpoint Inhibitors in Cancer Immunotherapy.Front Med (Lausanne). 2021 Nov 4;8:762247. doi: 10.3389/fmed.2021.762247. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34805229 Free PMC article. Review.
-
Evaluation of Cardiac Adverse Events with Nivolumab Using a Japanese Real-World Database.Clin Drug Investig. 2023 Mar;43(3):177-184. doi: 10.1007/s40261-023-01246-x. Epub 2023 Feb 13. Clin Drug Investig. 2023. PMID: 36780109
References
-
- Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J Immunother Cancer. 2017;5(1):95. doi: 10.1186/s40425-017-0300-z. - DOI - PMC - PubMed
-
- Raschi E, Gatti M, Gelsomino F, Ardizzoni A, Poluzzi E, De Ponti F. Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: a clinical perspective from Pharmacovigilance. Target Oncol. 2020;15(4):449–466. doi: 10.1007/s11523-020-00738-6. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

