Apabetalone and hospitalization for heart failure in patients following an acute coronary syndrome: a prespecified analysis of the BETonMACE study
- PMID: 33413345
- PMCID: PMC7791841
- DOI: 10.1186/s12933-020-01199-x
Apabetalone and hospitalization for heart failure in patients following an acute coronary syndrome: a prespecified analysis of the BETonMACE study
Abstract
Background: Patients with diabetes and acute coronary syndrome (ACS) are at high risk for subsequent heart failure. Apabetalone is a selective inhibitor of bromodomain and extra-terminal (BET) proteins, epigenetic regulators of gene expression. Preclinical data suggest that apabetalone exerts favorable effects on pathways related to myocardial structure and function and therefore could impact subsequent heart failure events. The effect of apabetalone on heart failure events after an ACS is not currently known.
Methods: The phase 3 BETonMACE trial was a double-blind, randomized comparison of apabetalone versus placebo on the incidence of major adverse cardiovascular events (MACE) in 2425 patients with a recent ACS and diabetes. This prespecified secondary analysis investigated the impact of apabetalone on hospitalization for congestive heart failure, not previously studied.
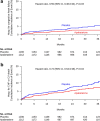
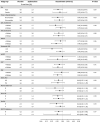
Results: Patients (age 62 years, 74.4% males, 90% high-intensity statin use, LDL-C 70.3 mg/dL, HDL-C 33.3 mg/dL and HbA1c 7.3%) were followed for an average 26 months. Apabetalone treated patients experienced the nominal finding of a lower rate of first hospitalization for heart failure (2.4% vs. 4.0%, HR 0.59 [95%CI 0.38-0.94], P = 0.03), total number of hospitalizations for heart failure (35 vs. 70, HR 0.47 [95%CI 0.27-0.83], P = 0.01) and the combination of cardiovascular death or hospitalization for heart failure (5.7% vs. 7.8%, HR 0.72 [95%CI 0.53-0.98], P = 0.04).
Conclusion: Apabetalone treatment was associated with fewer hospitalizations for heart failure in patients with type 2 diabetes and recent ACS. Future studies are warranted to define the potential for BET inhibition with apabetalone to prevent heart failure in patients with diabetes and ACS.
Keywords: Acute coronary syndrome; Atherosclerosis; BET inhibitors; Cardiovascular disease; Clinical trial; Diabetes; Epigenetics; Heart failure.
Conflict of interest statement
SJN reports research grants from AstraZeneca, Amgen, Anthera, Eli Lilly, Esperion, Novartis, Cerenis, The Medicines Company, Resverlogix, InfraReDx, Roche, Sanofi-Regeneron and LipoScience and honoraria from AstraZeneca, Akcea, Eli Lilly, Anthera, Omthera, Merck, Takeda, Resverlogix, Sanofi-Regeneron, CSL Behring, Esperion, Boehringer Ingelheim. GGS reports grants to his institution from Resverlogix, Roche, Sanofi and The Medicines Company. GGS also has a pending patent US 62/806,313 "Methods for Reducing Cardiovascular Risk" assigned in full to the University of Colorado. KAB reports research grants from Resverlogix, The Medicines Company, GlaxoSmithKline, Pfizer, BioCardia, Amgen and Cytokinetics. HNG reports honoraria from Resverlogix. JOJ, EK, NW and MS are employees of Resverlogix. KK-Z reports honoraria from Abbott, Abbvie, Alexion, Amgen, AstraZeneca, Aveo, Chugai, DaVita, Fresenius Medical Services, Genentech, Haymarket, Hospira, Kabi, Keryx, Novartis, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Vifor, ZS-Pharma, UpToDate, Baxter, Dr Schaer, Amag Pharma and grants from Shire, PCORI and NIH. PPT reports honoraria from Resverlogix, Amarin, Amgen, Kowa, Merck, Novo-Nordisk, Regeneron, Sanofi, Theravance. KKR reports honoraria from Resverlogix, Aegerion, Amgen, Pfizer, AstraZeneca, Cerenis, Akcea, The Medicines Company, Kowa, Novartis, Cipla, Eli Lilly, Algorithm, Takeda, Boehringer Ingelheim, Abbvie, Silence Therapeutics, Dr Reddys, Bayer, Daiichi Sankyo, Esperion, Zuelling Pharma, Sanofi-Regeneron and Merck and grants from Sanofi-Regeneron and Merck.
Figures
References
-
- Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, Deswal A, Dickson VV, Kosiborod MN, Lekavich CL, McCoy RG, Mentz RJ, Pina IL, American Heart Association Heart F, Transplantation Committee of the Council on Clinical C, Council on C, Stroke N and the Heart Failure Society of A Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140:e294–e324. doi: 10.1161/CIR.0000000000000691. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

