MicroRNA regulation of cancer stem cells in the pathogenesis of breast cancer
- PMID: 33413418
- PMCID: PMC7792222
- DOI: 10.1186/s12935-020-01716-8
MicroRNA regulation of cancer stem cells in the pathogenesis of breast cancer
Abstract
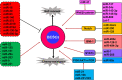
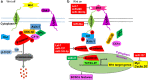
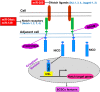
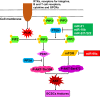
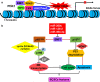
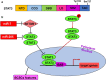
Breast cancer is the most common cancer among women and accounts for 30% of all female malignancies worldwide. Breast cancer stem cells (BCSCs) are a small population of breast cancer cells that exhibit multiple characteristics including differentiation capacity, self-renewal and therapeutic resistance. Recently, BCSCs have attracted attention due to their modulation of breast tumor behaviors and drug resistance. miRNAs are small noncoding mRNAs involved in virtually all biological processes, including stem cell development, maintenance and differentiation. In breast cancer, miRNAs appear to be multi-faceted since they can act as either suppressors or oncogenes to regulate breast cancer progression. This review summarizes the critical roles of miRNAs in regulating multiple signaling pathways such as Wnt/β-catenin, Notch, PI3K/AKT/mTOR, BMI-1 and STAT3 that are important for the BCSC maintenance.
Keywords: Breast cancer; Breast cancer stem cell (BCSC); Self-renewal; Therapeutic resistance; miRNA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Effects of miRNAs on functions of breast cancer stem cells and treatment of breast cancer.Onco Targets Ther. 2018 Jul 24;11:4263-4270. doi: 10.2147/OTT.S165156. eCollection 2018. Onco Targets Ther. 2018. PMID: 30100733 Free PMC article. Review.
-
MicroRNAs, a subpopulation of regulators, are involved in breast cancer progression through regulating breast cancer stem cells.Oncol Lett. 2017 Nov;14(5):5069-5076. doi: 10.3892/ol.2017.6867. Epub 2017 Sep 1. Oncol Lett. 2017. PMID: 29142594 Free PMC article.
-
Mechanistic Pathways of Malignancy in Breast Cancer Stem Cells.Front Oncol. 2020 Apr 30;10:452. doi: 10.3389/fonc.2020.00452. eCollection 2020. Front Oncol. 2020. PMID: 32426267 Free PMC article. Review.
-
Signaling pathways governing breast cancer stem cells behavior.Stem Cell Res Ther. 2021 Apr 16;12(1):245. doi: 10.1186/s13287-021-02321-w. Stem Cell Res Ther. 2021. PMID: 33863385 Free PMC article. Review.
-
Breast cancer stem cells, EMT and therapeutic targets.Biochem Biophys Res Commun. 2014 Oct 10;453(1):112-6. doi: 10.1016/j.bbrc.2014.09.069. Epub 2014 Sep 26. Biochem Biophys Res Commun. 2014. PMID: 25261721 Review.
Cited by
-
Regulatory Functions of microRNAs in Cancer Stem Cells: Mechanism, Facts, and Perspectives.Cells. 2025 Jul 14;14(14):1073. doi: 10.3390/cells14141073. Cells. 2025. PMID: 40710326 Free PMC article. Review.
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
-
Helicobacter pylori-activated fibroblasts as a silent partner in gastric cancer development.Cancer Metastasis Rev. 2023 Dec;42(4):1219-1256. doi: 10.1007/s10555-023-10122-1. Epub 2023 Jul 17. Cancer Metastasis Rev. 2023. PMID: 37460910 Free PMC article. Review.
-
The role of the mTOR pathway in breast cancer stem cells (BCSCs): mechanisms and therapeutic potentials.Stem Cell Res Ther. 2025 Mar 29;16(1):156. doi: 10.1186/s13287-025-04218-4. Stem Cell Res Ther. 2025. PMID: 40158191 Free PMC article. Review.
-
Expression pattern, prognostic value and potential microRNA silencing of FZD8 in breast cancer.Oncol Lett. 2023 Sep 21;26(5):477. doi: 10.3892/ol.2023.14065. eCollection 2023 Nov. Oncol Lett. 2023. PMID: 37809047 Free PMC article.
References
-
- Dong H, Diao H, Zhao Y, Xu H, Pei S, Gao J, Wang J, Hussain T, Zhao D, Zhou X, et al. Overexpression of matrix metalloproteinase-9 in breast cancer cell lines remarkably increases the cell malignancy largely via activation of transforming growth factor beta/SMAD signalling. Cell Proliferation. 2019;52(5):e12633. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

