Rationale and design of the Novel Uses of adaptive Designs to Guide provider Engagement in Electronic Health Records (NUDGE-EHR) pragmatic adaptive randomized trial: a trial protocol
- PMID: 33413494
- PMCID: PMC7792313
- DOI: 10.1186/s13012-020-01078-9
Rationale and design of the Novel Uses of adaptive Designs to Guide provider Engagement in Electronic Health Records (NUDGE-EHR) pragmatic adaptive randomized trial: a trial protocol
Abstract
Background: The prescribing of high-risk medications to older adults remains extremely common and results in potentially avoidable health consequences. Efforts to reduce prescribing have had limited success, in part because they have been sub-optimally timed, poorly designed, or not provided actionable information. Electronic health record (EHR)-based tools are commonly used but have had limited application in facilitating deprescribing in older adults. The objective is to determine whether designing EHR tools using behavioral science principles reduces inappropriate prescribing and clinical outcomes in older adults.
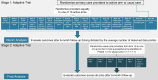
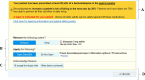
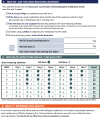
Methods: The Novel Uses of Designs to Guide provider Engagement in Electronic Health Records (NUDGE-EHR) project uses a two-stage, 16-arm adaptive randomized pragmatic trial with a "pick-the-winner" design to identify the most effective of many potential EHR tools among primary care providers and their patients ≥ 65 years chronically using benzodiazepines, sedative hypnotic ("Z-drugs"), or anticholinergics in a large integrated delivery system. In stage 1, we randomized providers and their patients to usual care (n = 81 providers) or one of 15 EHR tools (n = 8 providers per arm) designed using behavioral principles including salience, choice architecture, or defaulting. After 6 months of follow-up, we will rank order the arms based upon their impact on the trial's primary outcome (for both stages): reduction in inappropriate prescribing (via discontinuation or tapering). In stage 2, we will randomize (a) stage 1 usual care providers in a 1:1 ratio to one of the up to 5 most promising stage 1 interventions or continue usual care and (b) stage 1 providers in the unselected arms in a 1:1 ratio to one of the 5 most promising interventions or usual care. Secondary and tertiary outcomes include quantities of medication prescribed and utilized and clinically significant adverse outcomes.
Discussion: Stage 1 launched in October 2020. We plan to complete stage 2 follow-up in December 2021. These results will advance understanding about how behavioral science can optimize EHR decision support to improve prescribing and health outcomes. Adaptive trials have rarely been used in implementation science, so these findings also provide insight into how trials in this field could be more efficiently conducted.
Trial registration: Clinicaltrials.gov ( NCT04284553 , registered: February 26, 2020).
Keywords: Adaptive trial; Decision support; Deprescribing; Older adults; Pragmatic trial; Prescribing.
Conflict of interest statement
The investigators report no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

