Establishing a deeper understanding of the osteogenic differentiation of monolayer cultured human pluripotent stem cells using novel and detailed analyses
- PMID: 33413612
- PMCID: PMC7792045
- DOI: 10.1186/s13287-020-02085-9
Establishing a deeper understanding of the osteogenic differentiation of monolayer cultured human pluripotent stem cells using novel and detailed analyses
Abstract
Background: Derivation of osteoblast-like cells from human pluripotent stem cells (hPSCs) is a popular topic in bone tissue engineering. Although many improvements have been achieved, the low induction efficiency because of spontaneous differentiation hampers their applications. To solve this problem, a detailed understanding of the osteogenic differentiation process of hPSCs is urgently needed.
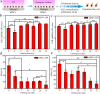
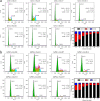
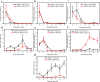
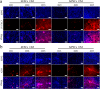
Methods: Monolayer cultured human embryonic stem cells and human-induced pluripotent stem cells were differentiated in commonly applied serum-containing osteogenic medium for 35 days. In addition to traditional assays such as cell viability detection, reverse transcription-polymerase chain reaction, immunofluorescence, and alizarin red staining, we also applied studies of cell counting, cell telomerase activity, and flow cytometry as essential indicators to analyse the cell type changes in each week.
Results: The population of differentiated cells was quite heterogeneous throughout the 35 days of induction. Then, cell telomerase activity and cell cycle analyses have value in evaluating the cell type and tumourigenicity of the obtained cells. Finally, a dynamic map was made to integrate the analysis of these results during osteogenic differentiation of hPSCs, and the cell types at defined stages were concluded.
Conclusions: Our results lay the foundation to improve the in vitro osteogenic differentiation efficiency of hPSCs by supplementing with functional compounds at the desired stage, and then establishing a stepwise induction system in the future.
Keywords: Human embryonic stem cells; Human-induced pluripotent stem cells; Marker expression; Osteogenic differentiation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Define of Optimal Addition Period of Osteogenic Peptide to Accelerate the Osteogenic Differentiation of Human Pluripotent Stem Cells.Tissue Eng Regen Med. 2024 Feb;21(2):291-308. doi: 10.1007/s13770-023-00597-y. Epub 2023 Oct 30. Tissue Eng Regen Med. 2024. PMID: 37903982 Free PMC article.
-
In vitro culture and directed osteogenic differentiation of human pluripotent stem cells on peptides-decorated two-dimensional microenvironment.ACS Appl Mater Interfaces. 2015 Mar 4;7(8):4560-72. doi: 10.1021/acsami.5b00188. Epub 2015 Feb 18. ACS Appl Mater Interfaces. 2015. PMID: 25671246
-
Conditioned Medium Enhances Osteogenic Differentiation of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells.Tissue Eng Regen Med. 2019 Jan 29;16(2):141-150. doi: 10.1007/s13770-018-0173-3. eCollection 2019 Apr. Tissue Eng Regen Med. 2019. PMID: 30989041 Free PMC article.
-
Induced Pluripotent Stem Cells as a new Strategy for Osteogenesis and Bone Regeneration.Stem Cell Rev Rep. 2015 Aug;11(4):645-51. doi: 10.1007/s12015-015-9594-8. Stem Cell Rev Rep. 2015. PMID: 26022504 Review.
-
Back to the future: lessons from development drive innovation of human pluripotent stem cell therapies.Exp Hematol. 2023 Jan;117:9-14. doi: 10.1016/j.exphem.2022.11.003. Epub 2022 Nov 16. Exp Hematol. 2023. PMID: 36400313 Review. No abstract available.
Cited by
-
Moringa oleifera L. Nanosuspension Extract Administration Affects Heat Shock Protein-10 and -70 under Orthodontics Mechanical Force In Vivo.Eur J Dent. 2025 May;19(2):523-530. doi: 10.1055/s-0044-1791937. Epub 2025 Jan 9. Eur J Dent. 2025. PMID: 39788532 Free PMC article.
-
Validation of a color deconvolution method to quantify MSC tri-lineage differentiation across species.Front Vet Sci. 2022 Oct 13;9:987045. doi: 10.3389/fvets.2022.987045. eCollection 2022. Front Vet Sci. 2022. PMID: 36311666 Free PMC article.
-
Antioxidant 1,2,3,4,6-Penta-O-galloyl-β-D-glucose Alleviating Apoptosis and Promoting Bone Formation Is Associated with Estrogen Receptors.Molecules. 2024 Oct 29;29(21):5110. doi: 10.3390/molecules29215110. Molecules. 2024. PMID: 39519751 Free PMC article.
-
Donor age and breed determine mesenchymal stromal cell characteristics.Stem Cell Res Ther. 2025 Feb 28;16(1):99. doi: 10.1186/s13287-025-04236-2. Stem Cell Res Ther. 2025. PMID: 40022193 Free PMC article.
-
Single-layer graphene oxide nanosheets induce proliferation and Osteogenesis of single-cell hBMSCs encapsulated in Alginate Microgels.Sci Rep. 2024 Oct 25;14(1):25272. doi: 10.1038/s41598-024-76957-y. Sci Rep. 2024. PMID: 39455695 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

