Biomarker testing in MCI patients-deciding who to test
- PMID: 33413634
- PMCID: PMC7792312
- DOI: 10.1186/s13195-020-00763-7
Biomarker testing in MCI patients-deciding who to test
Abstract
Background: We aimed to derive an algorithm to define the optimal proportion of patients with mild cognitive impairment (MCI) in whom cerebrospinal fluid (CSF) testing is of added prognostic value.
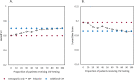
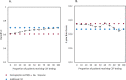
Methods: MCI patients were selected from the Amsterdam Dementia Cohort (n = 402). Three-year progression probabilities to dementia were predicted using previously published models with and without CSF data (amyloid-beta1-42 (Abeta), phosphorylated tau (p-tau)). We incrementally augmented the proportion of patients undergoing CSF, starting with the 10% patients with prognostic probabilities based on clinical data around the median (percentile 45-55), until all patients received CSF. The optimal proportion was defined as the proportion where the stepwise algorithm showed similar prognostic discrimination (Harrell's C) and accuracy (three-year Brier scores) compared to CSF testing of all patients. We used the BioFINDER study (n = 221) for validation.
Results: The optimal proportion of MCI patients to receive CSF testing selected by the stepwise approach was 50%. CSF testing in only this proportion improved the performance of the model with clinical data only from Harrell's C = 0.60, Brier = 0.198 (Harrell's C = 0.61, Brier = 0.197 if the information on magnetic resonance imaging was available) to Harrell's C = 0.67 and Brier = 0.190, and performed similarly to a model in which all patients received CSF testing. Applying the stepwise approach in the BioFINDER study would again select half of the MCI patients and yielded robust results with respect to prognostic performance.
Interpretation: CSF biomarker testing adds prognostic value in half of the MCI patients. As such, we achieve a CSF saving recommendation while simultaneously retaining optimal prognostic accuracy.
Keywords: Biomarkers; Decision support; MCI.
Conflict of interest statement
Ingrid S. van Maurik reports no financial disclosures or conflicts of interest.
Hanneke F.M. Rhodius-Meester performs contract research for Combinostics, and all funding is paid to her institution.
Charlotte E. Teunissen has a collaboration contract with ADx Neurosciences and Quanterix, performed contract research, or received grants from AxonNeurosciences, Biogen, Boehringer, Brainstorm Therapeutics, EIP farma, Esai, Janssen prevention center, Roche, Toyama, Vivoryon.
Philip Scheltens reports, outside the submitted work, support from Novartis NOVARTIS, GENENTECH, AC IMMUNE, AXON NEUROSCIENCE, EIP PHARMA, COGRX GEMVAX, CORTEXZYME, GREEN VALLEY, COGNOPTIX.
P.S. has acquired grant support (for the institution) from GE Healthcare, Danone Research, Piramal and Merck. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, GE Healthcare, Novartis, Sanofi, Nutricia, Probiodrug, Biogen, Roche, Avraham, and EIP Pharma.
Frederik Barkhof is supported by the NIHR biomedical research centre at UCLH.
Sebastian Palmqvist reports no financial disclosures or conflicts of interest.
Oskar Hansson has acquired research support (for the institution) from Roche, Pfizer, GE Healthcare, Biogen, Eli Lilly, and AVID Radiopharmaceuticals. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Biogen and Roche.
Wiesje M. van der Flier: Research programs of Wiesje van der Flier have been funded by ZonMW, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Pasman stichting, Biogen MA Inc., Boehringer Ingelheim, Life-MI, AVID, Roche BV, Fujifilm, Combinostics. WF holds the Pasman chair. WF has performed contract research for Biogen MA Inc. and Boehringer Ingelheim. WF has been an invited speaker at Boehringer Ingelheim and Biogen MA Inc. All funding is paid to her institution.
Johannes Berkhof reports no financial disclosures or conflicts of interest.
Figures
References
-
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

