Impact of a multimedia website with patient experiences of multiple sclerosis (PExMS) on immunotherapy decision-making: study protocol for a pilot randomised controlled trial in a mixed-methods design
- PMID: 33413658
- PMCID: PMC7788927
- DOI: 10.1186/s40814-020-00749-0
Impact of a multimedia website with patient experiences of multiple sclerosis (PExMS) on immunotherapy decision-making: study protocol for a pilot randomised controlled trial in a mixed-methods design
Abstract
Background: A variety of management options (e.g. immunotherapies, lifestyle interventions, and rehabilitation) are available for people with relapsing-remitting multiple sclerosis (RRMS). Besides coping with the diagnosis, people with MS (pwMS) have to make complex decisions such as deciding about immunotherapies. In addition to factual information, reports of patient experiences (PEx) may support patients in decision-making. The added value of PEx in decision-making is not clear, and controlled studies are rare. Therefore, systematic methods are necessary to develop and analyse PEx. As there are no evaluated PEx for MS in Germany, we are currently creating a website presenting PEx structured according to topics and illustrated by video, audio, and text files. We aim to determine the feasibility of an intervention using PEx and evaluate whether PEx may help pwMS in their immunotherapy decision-making processes as a supplement to evidence-based information.
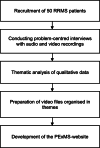
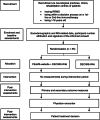
Methods: This project will follow the Medical Research Council framework for development and evaluation of complex interventions. After the development of a website with PEx, a randomised controlled pilot trial (pilot RCT) will be conducted in 2-3 MS centres, clinics, or rehabilitation centres including 55 pwMS and accompanied by a process evaluation. Patients with a RRMS diagnosis considering immunotherapy are eligible. The primary outcome is decision self-efficacy. Secondary outcomes include preparation for decision-making, decisional conflict, risk knowledge, confidence in active participation, affective forecasting, social support, and self-reported impact of eHealth on its users. Participants will be randomly assigned either to (i) an intervention group with 4 weeks access to an evidence-based patient information resource and the PExMS-website as an adjunct or to (ii) the control group with access to evidence-based information alone. A 6-member advisory panel involving representatives of pwMS, researchers, and neurologists, who accompany the whole project, will mentor this pilot RCT.
Discussion: The intervention was developed with systematic methods, created with active patient involvement and in critical appraisal by an expert advisory panel. The study is innovative as it contributes to the controversial evidence on the use of PEx in the context of evidence-based patient information.
Trial registration: ClinicalTrials.gov, NCT04236544.
Keywords: Decision support; Decision-making; Multiple sclerosis; Narrative information; Patient experiences; Web-based experiential information.
Conflict of interest statement
AB has received funding from Roche Pharma. CH has received research grants, congress travel compensations, and salaries for talks from Biogen, Genzyme, Sanofi-Aventis, Bayer Healthcare, Merck, Teva Pharma, Roche Pharma, and Novartis. IK has received speaker honoraria and travel funding from Bayer, Biogen, Novartis, Merck, Sanofi Genzyme, Roche; speaker honoraria from Mylan; travel funding from the Guthy-Jackson Charitable Foundation; consulted for Alexion, Bayer, Biogen, Celgene, Chugai, IQVIA, Novartis, Merck, Roche; and received research support from Chugai, Diamed. KRL, CK, JS, DE, SK, and JK declare that they have no competing interests. ACR was supported by a research grant from the National MS Society, USA (grant no. G-1508-06034).
Figures
References
-
- WHO . Atlas multiple sclerosis resources in the world 2008. Geneva; London: World Health Organization; Multiple Sclerosis International Federation; 2008. p. 51.
-
- DMSG. Was ist Multiple Sklerose?: Häufigkeit der MS 2017. Available from: https://www.dmsg.de/multiple-sklerose-infos/was-ist-ms/. Accessed May 2020.
-
- Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet (London, England) 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials