The diversity and breadth of cancer cell fatty acid metabolism
- PMID: 33413672
- PMCID: PMC7791669
- DOI: 10.1186/s40170-020-00237-2
The diversity and breadth of cancer cell fatty acid metabolism
Abstract
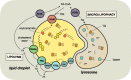
Tumor cellular metabolism exhibits distinguishing features that collectively enhance biomass synthesis while maintaining redox balance and cellular homeostasis. These attributes reflect the complex interactions between cell-intrinsic factors such as genomic-transcriptomic regulation and cell-extrinsic influences, including growth factor and nutrient availability. Alongside glucose and amino acid metabolism, fatty acid metabolism supports tumorigenesis and disease progression through a range of processes including membrane biosynthesis, energy storage and production, and generation of signaling intermediates. Here, we highlight the complexity of cellular fatty acid metabolism in cancer, the various inputs and outputs of the intracellular free fatty acid pool, and the numerous ways that these pathways influence disease behavior.
Keywords: Cellular membrane; De novo synthesis; Fatty acid; Lipid; Lipid droplets; Mitochondria; Oxidation; Peroxisome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Control of energy homeostasis: role of enzymes and intermediates of fatty acid metabolism in the central nervous system.Annu Rev Nutr. 2006;26:23-44. doi: 10.1146/annurev.nutr.25.050304.092532. Annu Rev Nutr. 2006. PMID: 16704352 Review.
-
Dietary fats and membrane function: implications for metabolism and disease.Biol Rev Camb Philos Soc. 2005 Feb;80(1):155-69. doi: 10.1017/s1464793104006578. Biol Rev Camb Philos Soc. 2005. PMID: 15727042 Review.
-
Impact of forced fatty acid synthesis on metabolism and physiology of Saccharomyces cerevisiae.FEMS Yeast Res. 2018 Dec 1;18(8). doi: 10.1093/femsyr/foy096. FEMS Yeast Res. 2018. PMID: 30169781
-
Lipid Droplets and the Management of Cellular Stress.Yale J Biol Med. 2019 Sep 20;92(3):435-452. eCollection 2019 Sep. Yale J Biol Med. 2019. PMID: 31543707 Free PMC article. Review.
-
Free radical biology for medicine: learning from nonalcoholic fatty liver disease.Free Radic Biol Med. 2013 Dec;65:952-968. doi: 10.1016/j.freeradbiomed.2013.08.174. Epub 2013 Aug 29. Free Radic Biol Med. 2013. PMID: 23994574 Review.
Cited by
-
Leveraging altered lipid metabolism in treating B cell malignancies.Prog Lipid Res. 2024 Jul;95:101288. doi: 10.1016/j.plipres.2024.101288. Epub 2024 Jul 2. Prog Lipid Res. 2024. PMID: 38964473 Free PMC article. Review.
-
Effect of Short-Chain Fatty Acids and Polyunsaturated Fatty Acids on Metabolites in H460 Lung Cancer Cells.Molecules. 2023 Mar 3;28(5):2357. doi: 10.3390/molecules28052357. Molecules. 2023. PMID: 36903601 Free PMC article.
-
Aster glehni F. Schmidt Extract Modulates the Activities of HMG-CoA Reductase and Fatty Acid Synthase.Plants (Basel). 2021 Oct 25;10(11):2287. doi: 10.3390/plants10112287. Plants (Basel). 2021. PMID: 34834649 Free PMC article.
-
Envisioning Glucose Transporters (GLUTs and SGLTs) as Novel Intervention against Cancer: Drug Discovery Perspective and Targeting Approach.Curr Drug Targets. 2025;26(2):109-131. doi: 10.2174/0113894501335877240926101134. Curr Drug Targets. 2025. PMID: 39377414 Review.
-
Untargeted metabolomic profiling of serum and urine in kidney cancer: a non-invasive approach for biomarker discovery.Metabolomics. 2025 Jul 1;21(4):97. doi: 10.1007/s11306-025-02294-4. Metabolomics. 2025. PMID: 40593405 Free PMC article.
References
-
- Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017;5:1. doi: 10.1186/s40170-016-0163-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

