14-3-3 mitigates alpha-synuclein aggregation and toxicity in the in vivo preformed fibril model
- PMID: 33413679
- PMCID: PMC7792107
- DOI: 10.1186/s40478-020-01110-5
14-3-3 mitigates alpha-synuclein aggregation and toxicity in the in vivo preformed fibril model
Abstract
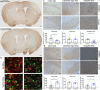
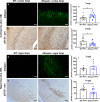
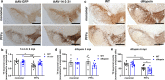
Alpha-synuclein (αsyn) is the key component of proteinaceous aggregates termed Lewy Bodies that pathologically define a group of disorders known as synucleinopathies, including Parkinson's Disease (PD) and Dementia with Lewy Bodies. αSyn is hypothesized to misfold and spread throughout the brain in a prion-like fashion. Transmission of αsyn necessitates the release of misfolded αsyn from one cell and the uptake of that αsyn by another, in which it can template the misfolding of endogenous αsyn upon cell internalization. 14-3-3 proteins are a family of highly expressed brain proteins that are neuroprotective in multiple PD models. We have previously shown that 14-3-3θ acts as a chaperone to reduce αsyn aggregation, cell-to-cell transmission, and neurotoxicity in the in vitro pre-formed fibril (PFF) model. In this study, we expanded our studies to test the impact of 14-3-3s on αsyn toxicity in the in vivo αsyn PFF model. We used both transgenic expression models and adenovirus associated virus (AAV)-mediated expression to examine whether 14-3-3 manipulation impacts behavioral deficits, αsyn aggregation, and neuronal counts in the PFF model. 14-3-3θ transgene overexpression in cortical and amygdala regions rescued social dominance deficits induced by PFFs at 6 months post injection, whereas 14-3-3 inhibition by transgene expression of the competitive 14-3-3 peptide inhibitor difopein in the cortex and amygdala accelerated social dominance deficits. The behavioral rescue by 14-3-3θ overexpression was associated with delayed αsyn aggregation induced by PFFs in these brain regions. Conversely, 14-3-3 inhibition by difopein in the cortex and amygdala accelerated αsyn aggregation and reduction in NECAB1-positive neuron counts induced by PFFs. 14-3-3θ overexpression by AAV in the substantia nigra (SN) also delayed αsyn aggregation in the SN and partially rescued PFF-induced reduction in tyrosine hydroxylase (TH)-positive dopaminergic cells in the SN. 14-3-3 inhibition in the SN accelerated nigral αsyn aggregation and enhanced PFF-induced reduction in TH-positive dopaminergic cells. These data indicate a neuroprotective role for 14-3-3θ against αsyn toxicity in vivo.
Keywords: 14-3-3s; Alpha-synuclein; Amygdala; Cortex; Dementia with Lewy Bodies; Mouse; Parkinson’s disease; Substantia nigra.
Conflict of interest statement
Dr. Yacoubian has a U.S. Patent No. 7,919,262 on the use of 14-3-3s in neurodegeneration. The remaining authors have no competing interests to declare.
Figures


Similar articles
-
14-3-3θ phosphorylation exacerbates alpha-synuclein aggregation and toxicity.Neurobiol Dis. 2025 Mar;206:106801. doi: 10.1016/j.nbd.2025.106801. Epub 2025 Jan 11. Neurobiol Dis. 2025. PMID: 39805369 Free PMC article.
-
14-3-3 Proteins Reduce Cell-to-Cell Transfer and Propagation of Pathogenic α-Synuclein.J Neurosci. 2018 Sep 19;38(38):8211-8232. doi: 10.1523/JNEUROSCI.1134-18.2018. Epub 2018 Aug 9. J Neurosci. 2018. PMID: 30093536 Free PMC article.
-
Loss of One Engrailed1 Allele Enhances Induced α-Synucleinopathy.J Parkinsons Dis. 2019;9(2):315-326. doi: 10.3233/JPD-191590. J Parkinsons Dis. 2019. PMID: 30932894 Free PMC article.
-
Initiation and propagation of α-synuclein aggregation in the nervous system.Mol Neurodegener. 2020 Mar 6;15(1):19. doi: 10.1186/s13024-020-00368-6. Mol Neurodegener. 2020. PMID: 32143659 Free PMC article. Review.
-
The emerging role of α-synuclein truncation in aggregation and disease.J Biol Chem. 2020 Jul 24;295(30):10224-10244. doi: 10.1074/jbc.REV120.011743. Epub 2020 May 18. J Biol Chem. 2020. PMID: 32424039 Free PMC article. Review.
Cited by
-
Cohort-specific boolean models highlight different regulatory modules during Parkinson's disease progression.iScience. 2024 Sep 14;27(10):110956. doi: 10.1016/j.isci.2024.110956. eCollection 2024 Oct 18. iScience. 2024. PMID: 39429779 Free PMC article.
-
Physiological Consequences of Targeting 14-3-3 and Its Interacting Partners in Neurodegenerative Diseases.Int J Mol Sci. 2022 Dec 7;23(24):15457. doi: 10.3390/ijms232415457. Int J Mol Sci. 2022. PMID: 36555098 Free PMC article.
-
A-Syn(ful) MAM: A Fresh Perspective on a Converging Domain in Parkinson's Disease.Int J Mol Sci. 2024 Jun 13;25(12):6525. doi: 10.3390/ijms25126525. Int J Mol Sci. 2024. PMID: 38928232 Free PMC article. Review.
-
Prospective Role of PAK6 and 14-3-3γ as Biomarkers for Parkinson's Disease.J Parkinsons Dis. 2024;14(3):495-506. doi: 10.3233/JPD-230402. J Parkinsons Dis. 2024. PMID: 38640169 Free PMC article.
-
Stoichiometric 14-3-3ζ binding promotes phospho-Tau microtubule dissociation and reduces aggregation and condensation.Commun Biol. 2025 Jul 31;8(1):1139. doi: 10.1038/s42003-025-08548-0. Commun Biol. 2025. PMID: 40745206 Free PMC article.
References
-
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. - DOI - PMC - PubMed
-
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. - DOI - PMC - PubMed
-
- Wang B, Underwood R, Kamath A, Britain C, McFerrin MB, McLean PJ, Volpicelli-Daley LA, Whitaker RH, Placzek WJ, Becker K, et al. 14-3-3 proteins reduce cell-to-cell transfer and propagation of pathogenic α-synuclein. J Neurosci. 2018;38:8211–8232. doi: 10.1523/jneurosci.1134-18.2018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

