Impact of mass testing during an epidemic rebound of SARS-CoV-2: a modelling study using the example of France
- PMID: 33413741
- PMCID: PMC7791601
- DOI: 10.2807/1560-7917.ES.2020.26.1.2001978
Impact of mass testing during an epidemic rebound of SARS-CoV-2: a modelling study using the example of France
Abstract
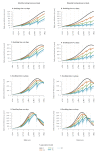
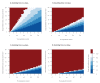
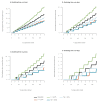
We used a mathematical model to evaluate the impact of mass testing in the control of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Under optimistic assumptions, one round of mass testing may reduce daily infections by up to 20-30%. Consequently, very frequent testing would be required to control a quickly growing epidemic if other control measures were to be relaxed. Mass testing is most relevant when epidemic growth remains limited through a combination of interventions.
Keywords: Covid-19; SARS-CoV-2; epidemic; mass testing.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
