ARID1A Mutation May Define an Immunologically Active Subgroup in Patients with Microsatellite Stable Colorectal Cancer
- PMID: 33414133
- PMCID: PMC7956157
- DOI: 10.1158/1078-0432.CCR-20-2404
ARID1A Mutation May Define an Immunologically Active Subgroup in Patients with Microsatellite Stable Colorectal Cancer
Abstract
Purpose: AT-rich interactive domain 1A (ARID1A) is commonly mutated in colorectal cancer, frequently resulting in truncation and loss of protein expression. ARID1A recruits MSH2 for mismatch repair during DNA replication. ARID1A deficiency promotes hypermutability and immune activation in preclinical models, but its role in patients with colorectal cancer is being explored.
Experimental design: The DNA sequencing and gene expression profiling of patients with colorectal cancer were extracted from The Cancer Genome Atlas and MD Anderson Cancer Center databases, with validation utilizing external databases, and correlation between ARID1A and immunologic features. IHC for T-cell markers was performed on a separate cohort of patients.
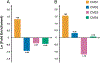
Results: Twenty-eight of 417 patients with microsatellite stable (MSS) colorectal cancer (6.7%) had ARID1A mutation. Among 58 genes most commonly mutated in colorectal cancer, ARID1A mutation had the highest increase with frameshift mutation rates in MSS cases (8-fold, P < 0.001). In MSS, ARID1A mutation was enriched in immune subtype (CMS1) and had a strong correlation with IFNγ expression (Δz score +1.91, P < 0.001). Compared with ARID1A wild-type, statistically significant higher expression for key checkpoint genes (e.g., PD-L1, CTLA4, and PDCD1) and gene sets (e.g., antigen presentation, cytotoxic T-cell function, and immune checkpoints) was observed in mutant cases. This was validated by unsupervised differential expression of genes related to immune response and further confirmed by higher infiltration of T cells in IHC of tumors with ARID1A mutation (P = 0.01).
Conclusions: The immunogenicity of ARID1A-mutant cases is likely due to an increased level of neoantigens resulting from increased tumor mutational burden and frameshift mutations. Tumors with ARID1A mutation may be more susceptible to immune therapy-based treatment strategies and should be recognized as a unique molecular subgroup in future immune therapy trials.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest:
The authors declare that they have no conflicts of interest.
Figures
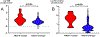
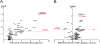


Similar articles
-
Cytolytic activity correlates with the mutational burden and deregulated expression of immune checkpoints in colorectal cancer.J Exp Clin Cancer Res. 2019 Aug 20;38(1):364. doi: 10.1186/s13046-019-1372-z. J Exp Clin Cancer Res. 2019. PMID: 31429779 Free PMC article.
-
The effects of ARID1A mutations on colorectal cancer and associations with PD-L1 expression by stromal cells.Cancer Rep (Hoboken). 2022 Jan;5(1):e1420. doi: 10.1002/cnr2.1420. Epub 2021 May 27. Cancer Rep (Hoboken). 2022. PMID: 34042312 Free PMC article.
-
The impact of ARID1A mutation on molecular characteristics in colorectal cancer.Eur J Cancer. 2020 Nov;140:119-129. doi: 10.1016/j.ejca.2020.09.006. Epub 2020 Oct 17. Eur J Cancer. 2020. PMID: 33080474 Free PMC article.
-
Is There a Role for Programmed Death Ligand-1 Testing and Immunotherapy in Colorectal Cancer With Microsatellite Instability? Part II-The Challenge of Programmed Death Ligand-1 Testing and Its Role in Microsatellite Instability-High Colorectal Cancer.Arch Pathol Lab Med. 2018 Jan;142(1):26-34. doi: 10.5858/arpa.2017-0041-RA. Epub 2017 Nov 9. Arch Pathol Lab Med. 2018. PMID: 29120224 Review.
-
ARID1A deficiency and immune checkpoint blockade therapy: From mechanisms to clinical application.Cancer Lett. 2020 Mar 31;473:148-155. doi: 10.1016/j.canlet.2020.01.001. Epub 2020 Jan 3. Cancer Lett. 2020. PMID: 31911080 Review.
Cited by
-
Expression of Autophagic and Inflammatory Markers in Normal Mucosa of Individuals with Colorectal Adenomas: A Cross Sectional Study among Italian Outpatients Undergoing Colonoscopy.Int J Mol Sci. 2022 May 6;23(9):5211. doi: 10.3390/ijms23095211. Int J Mol Sci. 2022. PMID: 35563601 Free PMC article.
-
Targeting ARID1A-Deficient Cancers: An Immune-Metabolic Perspective.Cells. 2023 Mar 21;12(6):952. doi: 10.3390/cells12060952. Cells. 2023. PMID: 36980292 Free PMC article. Review.
-
The effects of ARID1A mutation in gastric cancer and its significance for treatment.Cancer Cell Int. 2023 Nov 26;23(1):296. doi: 10.1186/s12935-023-03154-8. Cancer Cell Int. 2023. PMID: 38008753 Free PMC article. Review.
-
SWI/SNF complex alterations as a biomarker of immunotherapy efficacy in pancreatic cancer.JCI Insight. 2021 Sep 22;6(18):e150453. doi: 10.1172/jci.insight.150453. JCI Insight. 2021. PMID: 34375311 Free PMC article.
-
ARID1A Downregulation Predicts High PD-L1 Expression and Worse Clinical Outcome in Patients With Gallbladder Cancer.Front Oncol. 2022 Feb 7;12:787897. doi: 10.3389/fonc.2022.787897. eCollection 2022. Front Oncol. 2022. PMID: 35198440 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68(6):394–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

