COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice
- PMID: 33414159
- PMCID: PMC8092708
- DOI: 10.1128/JVI.02260-20
COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice
Abstract
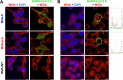
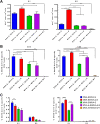
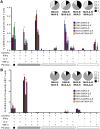
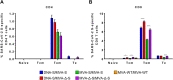
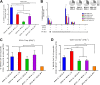
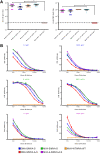
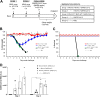
Vaccines against SARS-CoV-2, the causative agent of the COVID-19 pandemic, are urgently needed. We developed two COVID-19 vaccines based on modified vaccinia virus Ankara (MVA) vectors expressing the entire SARS-CoV-2 spike (S) protein (MVA-CoV2-S); their immunogenicity was evaluated in mice using DNA/MVA or MVA/MVA prime/boost immunizations. Both vaccines induced robust, broad and polyfunctional S-specific CD4+ (mainly Th1) and CD8+ T-cell responses, with a T effector memory phenotype. DNA/MVA immunizations elicited higher T-cell responses. All vaccine regimens triggered high titers of IgG antibodies specific for the S, as well as for the receptor-binding domain; the predominance of the IgG2c isotype was indicative of Th1 immunity. Notably, serum samples from vaccinated mice neutralized SARS-CoV-2 in cell cultures, and those from MVA/MVA immunizations showed a higher neutralizing capacity. Remarkably, one or two doses of MVA-CoV2-S protect humanized K18-hACE2 mice from a lethal dose of SARS-CoV-2. In addition, two doses of MVA-CoV2-S confer full inhibition of virus replication in the lungs. These results demonstrate the robust immunogenicity and full efficacy of MVA-based COVID-19 vaccines in animal models and support its translation to the clinic.IMPORTANCE The continuous dissemination of the novel emerging SARS-CoV-2 virus, with more than 78 million infected cases worldwide and higher than 1,700,000 deaths as of December 23, 2020, highlights the urgent need for the development of novel vaccines against COVID-19. With this aim, we have developed novel vaccine candidates based on the poxvirus modified vaccinia virus Ankara (MVA) strain expressing the full-length SARS-CoV-2 spike (S) protein, and we have evaluated their immunogenicity in mice using DNA/MVA or MVA/MVA prime/boost immunization protocols. The results showed the induction of a potent S-specific T-cell response and high titers of neutralizing antibodies. Remarkably, humanized K18-hACE2 mice immunized with one or two doses of the MVA-based vaccine were 100% protected from SARS-CoV-2 lethality. Moreover, two doses of the vaccine prevented virus replication in lungs. Our findings prove the robust immunogenicity and efficacy of MVA-based COVID-19 vaccines in animal models and support its translation to the clinic.
Copyright © 2021 American Society for Microbiology.
Figures


Similar articles
-
MVA-based vaccine candidates expressing SARS-CoV-2 prefusion-stabilized spike proteins of the Wuhan, Beta or Omicron BA.1 variants protect transgenic K18-hACE2 mice against Omicron infection and elicit robust and broad specific humoral and cellular immune responses.Front Immunol. 2024 Aug 29;15:1420304. doi: 10.3389/fimmu.2024.1420304. eCollection 2024. Front Immunol. 2024. PMID: 39267752 Free PMC article.
-
Intranasal administration of a single dose of MVA-based vaccine candidates against COVID-19 induced local and systemic immune responses and protects mice from a lethal SARS-CoV-2 infection.Front Immunol. 2022 Sep 12;13:995235. doi: 10.3389/fimmu.2022.995235. eCollection 2022. Front Immunol. 2022. PMID: 36172368 Free PMC article.
-
A Single Dose of an MVA Vaccine Expressing a Prefusion-Stabilized SARS-CoV-2 Spike Protein Neutralizes Variants of Concern and Protects Mice From a Lethal SARS-CoV-2 Infection.Front Immunol. 2022 Jan 27;12:824728. doi: 10.3389/fimmu.2021.824728. eCollection 2021. Front Immunol. 2022. PMID: 35154086 Free PMC article.
-
Past, Present, and Future of Viral Vector Vaccine Platforms: A Comprehensive Review.Vaccines (Basel). 2025 May 15;13(5):524. doi: 10.3390/vaccines13050524. Vaccines (Basel). 2025. PMID: 40432133 Free PMC article. Review.
-
Correlates of Vaccine-Induced Protection against SARS-CoV-2.Vaccines (Basel). 2021 Mar 10;9(3):238. doi: 10.3390/vaccines9030238. Vaccines (Basel). 2021. PMID: 33801831 Free PMC article. Review.
Cited by
-
Warmer ambient air temperatures reduce nasal turbinate and brain infection, but increase lung inflammation in the K18-hACE2 mouse model of COVID-19.Sci Total Environ. 2023 Feb 10;859(Pt 1):160163. doi: 10.1016/j.scitotenv.2022.160163. Epub 2022 Nov 14. Sci Total Environ. 2023. PMID: 36395835 Free PMC article.
-
Elevated levels of cell-free NKG2D-ligands modulate NKG2D surface expression and compromise NK cell function in severe COVID-19 disease.Front Immunol. 2024 Feb 12;15:1273942. doi: 10.3389/fimmu.2024.1273942. eCollection 2024. Front Immunol. 2024. PMID: 38410511 Free PMC article.
-
Single Immunization with Recombinant ACAM2000 Vaccinia Viruses Expressing the Spike and the Nucleocapsid Proteins Protects Hamsters against SARS-CoV-2-Caused Clinical Disease.J Virol. 2022 May 11;96(9):e0038922. doi: 10.1128/jvi.00389-22. Epub 2022 Apr 12. J Virol. 2022. PMID: 35412347 Free PMC article.
-
Cross-reactive cellular, but not humoral, immunity is detected between OC43 and SARS-CoV-2 NPs in people not infected with SARS-CoV-2: Possible role of cTFH cells.J Leukoc Biol. 2022 Aug;112(2):339-346. doi: 10.1002/JLB.4COVCRA0721-356RRR. Epub 2022 Apr 5. J Leukoc Biol. 2022. PMID: 35384035 Free PMC article.
-
Single-dose replicating poxvirus vector-based RBD vaccine drives robust humoral and T cell immune response against SARS-CoV-2 infection.Mol Ther. 2022 May 4;30(5):1885-1896. doi: 10.1016/j.ymthe.2021.10.008. Epub 2021 Oct 20. Mol Ther. 2022. PMID: 34687845 Free PMC article.
References
-
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–574. doi:10.1016/S0140-6736(20)30251-8. - DOI - PMC - PubMed
-
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team . 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

