Ataluren and aminoglycosides stimulate read-through of nonsense codons by orthogonal mechanisms
- PMID: 33414181
- PMCID: PMC7812769
- DOI: 10.1073/pnas.2020599118
Ataluren and aminoglycosides stimulate read-through of nonsense codons by orthogonal mechanisms
Abstract
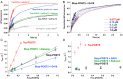
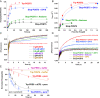
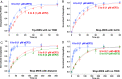
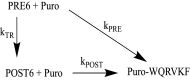
During protein synthesis, nonsense mutations, resulting in premature stop codons (PSCs), produce truncated, inactive protein products. Such defective gene products give rise to many diseases, including cystic fibrosis, Duchenne muscular dystrophy (DMD), and some cancers. Small molecule nonsense suppressors, known as TRIDs (translational read-through-inducing drugs), stimulate stop codon read-through. The best characterized TRIDs are ataluren, which has been approved by the European Medicines Agency for the treatment of DMD, and G418, a structurally dissimilar aminoglycoside. Previously [1], we applied a highly purified in vitro eukaryotic translation system to demonstrate that both aminoglycosides like G418 and more hydrophobic molecules like ataluren stimulate read-through by direct interaction with the cell's protein synthesis machinery. Our results suggested that they might do so by different mechanisms. Here, we pursue this suggestion through a more-detailed investigation of ataluren and G418 effects on read-through. We find that ataluren stimulation of read-through derives exclusively from its ability to inhibit release factor activity. In contrast, G418 increases functional near-cognate tRNA mispairing with a PSC, resulting from binding to its tight site on the ribosome, with little if any effect on release factor activity. The low toxicity of ataluren suggests that development of new TRIDs exclusively directed toward inhibiting termination should be a priority in combatting PSC diseases. Our results also provide rate measurements of some of the elementary steps during the eukaryotic translation elongation cycle, allowing us to determine how these rates are modified when cognate tRNA is replaced by near-cognate tRNA ± TRIDs.
Keywords: G418; TRID; ataluren; nonsense suppression; read-through.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


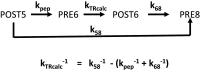
Similar articles
-
Strategies against Nonsense: Oxadiazoles as Translational Readthrough-Inducing Drugs (TRIDs).Int J Mol Sci. 2019 Jul 6;20(13):3329. doi: 10.3390/ijms20133329. Int J Mol Sci. 2019. PMID: 31284579 Free PMC article. Review.
-
Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression.Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12508-12513. doi: 10.1073/pnas.1605336113. Epub 2016 Oct 4. Proc Natl Acad Sci U S A. 2016. PMID: 27702906 Free PMC article.
-
Read-through strategies for suppression of nonsense mutations in Duchenne/ Becker muscular dystrophy: aminoglycosides and ataluren (PTC124).J Child Neurol. 2010 Sep;25(9):1158-64. doi: 10.1177/0883073810371129. Epub 2010 Jun 2. J Child Neurol. 2010. PMID: 20519671 Free PMC article. Review.
-
Stop codon context influences genome-wide stimulation of termination codon readthrough by aminoglycosides.Elife. 2020 Jan 23;9:e52611. doi: 10.7554/eLife.52611. Elife. 2020. PMID: 31971508 Free PMC article.
-
Small molecule Y-320 stimulates ribosome biogenesis, protein synthesis, and aminoglycoside-induced premature termination codon readthrough.PLoS Biol. 2021 May 3;19(5):e3001221. doi: 10.1371/journal.pbio.3001221. eCollection 2021 May. PLoS Biol. 2021. PMID: 33939688 Free PMC article.
Cited by
-
Small-molecule eRF3a degraders rescue CFTR nonsense mutations by promoting premature termination codon readthrough.J Clin Invest. 2022 Sep 15;132(18):e154571. doi: 10.1172/JCI154571. J Clin Invest. 2022. PMID: 35900863 Free PMC article.
-
Cardiovascular Disease in Duchenne Muscular Dystrophy: Overview and Insight Into Novel Therapeutic Targets.JACC Basic Transl Sci. 2022 Mar 9;7(6):608-625. doi: 10.1016/j.jacbts.2021.11.004. eCollection 2022 Jun. JACC Basic Transl Sci. 2022. PMID: 35818510 Free PMC article. Review.
-
Combining nonsense mutation suppression therapy with nonsense-mediated decay inhibition in neurofibromatosis type 1.Mol Ther Nucleic Acids. 2023 Jun 26;33:227-239. doi: 10.1016/j.omtn.2023.06.018. eCollection 2023 Sep 12. Mol Ther Nucleic Acids. 2023. PMID: 37520682 Free PMC article.
-
Single-Molecule Studies of Cognate and Near-Cognate Elongation in an in vitro Eukaryotic Translation System.bioRxiv [Preprint]. 2024 Aug 30:2024.08.29.609187. doi: 10.1101/2024.08.29.609187. bioRxiv. 2024. PMID: 39257735 Free PMC article. Preprint.
-
Novel Translational Read-through-Inducing Drugs as a Therapeutic Option for Shwachman-Diamond Syndrome.Biomedicines. 2022 Apr 12;10(4):886. doi: 10.3390/biomedicines10040886. Biomedicines. 2022. PMID: 35453634 Free PMC article.
References
-
- Brenner S., Stretton A. O. W., Kaplan S., Genetic code: The ‘nonsense’ triplets for chain termination and their suppression. Nature 206, 994–998 (1965). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

