Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes
- PMID: 33414264
- PMCID: PMC7797270
- DOI: 10.1136/jitc-2020-001731
Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes
Erratum in
-
Correction: Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes.J Immunother Cancer. 2022 Sep;10(9):e001731corr1. doi: 10.1136/jitc-2020-001731corr1. J Immunother Cancer. 2022. PMID: 36096536 Free PMC article. No abstract available.
-
Correction: Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes.J Immunother Cancer. 2023 Nov 29;11(11):e001731corr2. doi: 10.1136/jitc-2020-001731corr2. J Immunother Cancer. 2023. PMID: 38030305 Free PMC article. No abstract available.
Abstract
Background: Immune-checkpoint inhibitor (ICI)-pneumonitis that does not improve or resolve with corticosteroids and requires additional immunosuppression is termed steroid-refractory ICI-pneumonitis. Herein, we report the clinical features, management and outcomes for patients treated with intravenous immunoglobulin (IVIG), infliximab, or the combination of IVIG and infliximab for steroid-refractory ICI-pneumonitis.
Methods: Patients with steroid-refractory ICI-pneumonitis were identified between January 2011 and January 2020 at a tertiary academic center. ICI-pneumonitis was defined as clinical or radiographic lung inflammation without an alternative diagnosis, confirmed by a multidisciplinary team. Steroid-refractory ICI-pneumonitis was defined as lack of clinical improvement after high-dose corticosteroids for 48 hours, necessitating additional immunosuppression. Serial clinical, radiologic (CT imaging), and functional features (level-of-care, oxygen requirement) were collected preadditional and postadditional immunosuppression.
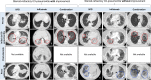
Results: Of 65 patients with ICI-pneumonitis, 18.5% (12/65) had steroid-refractory ICI-pneumonitis. Mean age at diagnosis of ICI-pneumonitis was 66.8 years (range: 35-85), 50% patients were male, and the majority had lung carcinoma (75%). Steroid-refractory ICI-pneumonitis occurred after a mean of 5 ICI doses from PD-(L)1 start (range: 3-12 doses). The most common radiologic pattern was diffuse alveolar damage (DAD: 50%, 6/12). After corticosteroid failure, patients were treated with: IVIG (n=7), infliximab (n=2), or combination IVIG and infliximab (n=3); 11/12 (91.7%) required ICU-level care and 8/12 (75%) died of steroid-refractory ICI-pneumonitis or infectious complications (IVIG alone=3/7, 42.9%; infliximab alone=2/2, 100%; IVIG + infliximab=3/3, 100%). All five patients treated with infliximab (5/5; 100%) died from steroid-refractory ICI-pneumonitis or infectious complications. Mechanical ventilation was required in 53% of patients treated with infliximab alone, 80% of those treated with IVIG + infliximab, and 25.5% of those treated with IVIG alone.
Conclusions: Steroid-refractory ICI-pneumonitis constituted 18.5% of referrals for multidisciplinary irAE care. Steroid-refractory ICI-pnuemonitis occurred early in patients' treatment courses, and most commonly exhibited a DAD radiographic pattern. Patients treated with IVIG alone demonstrated an improvement in both level-of-care and oxygenation requirements and had fewer fatalities (43%) from steroid-refractory ICI-pneumonitis when compared to treatment with infliximab (100% mortality).
Keywords: immunotherapy; inflammation; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Comment in
-
Real-world insights on preferred treatments for steroid-refractory immune checkpoint inhibitor-induced pneumonitis.J Immunother Cancer. 2021 Feb;9(2):e002252. doi: 10.1136/jitc-2020-002252. J Immunother Cancer. 2021. PMID: 33602697 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
