Drebrin regulates cytoskeleton dynamics in migrating neurons through interaction with CXCR4
- PMID: 33414275
- PMCID: PMC7826346
- DOI: 10.1073/pnas.2009493118
Drebrin regulates cytoskeleton dynamics in migrating neurons through interaction with CXCR4
Abstract
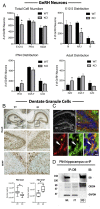
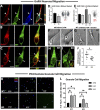
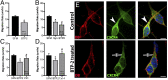
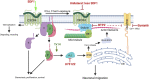
Stromal cell-derived factor-1 (SDF-1) and chemokine receptor type 4 (CXCR4) are regulators of neuronal migration (e.g., GnRH neurons, cortical neurons, and hippocampal granule cells). However, how SDF-1/CXCR4 alters cytoskeletal components remains unclear. Developmentally regulated brain protein (drebrin) stabilizes actin polymerization, interacts with microtubule plus ends, and has been proposed to directly interact with CXCR4 in T cells. The current study examined, in mice, whether CXCR4 under SDF-1 stimulation interacts with drebrin to facilitate neuronal migration. Bioinformatic prediction of protein-protein interaction highlighted binding sites between drebrin and crystallized CXCR4. In migrating GnRH neurons, drebrin, CXCR4, and the microtubule plus-end binding protein EB1 were localized close to the cell membrane. Coimmunoprecipitation (co-IP) confirmed a direct interaction between drebrin and CXCR4 using wild-type E14.5 whole head and a GnRH cell line. Analysis of drebrin knockout (DBN1 KO) mice showed delayed migration of GnRH cells into the brain. A decrease in hippocampal granule cells was also detected, and co-IP confirmed a direct interaction between drebrin and CXCR4 in PN4 hippocampi. Migration assays on primary neurons established that inhibiting drebrin (either pharmacologically or using cells from DBN1 KO mice) prevented the effects of SDF-1 on neuronal movement. Bioinformatic prediction then identified binding sites between drebrin and the microtubule plus end protein, EB1, and super-resolution microscopy revealed decreased EB1 and drebrin coexpression after drebrin inhibition. Together, these data show a mechanism by which a chemokine, via a membrane receptor, communicates with the intracellular cytoskeleton in migrating neurons during central nervous system development.
Keywords: CXCR4; GnRH; drebrin; hippocampus; neuronal migration.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
CXCR4/SDF-1 system modulates development of GnRH-1 neurons and the olfactory system.Dev Neurobiol. 2008 Mar;68(4):487-503. doi: 10.1002/dneu.20594. Dev Neurobiol. 2008. PMID: 18188864
-
The drebrin/EB3 pathway regulates cytoskeletal dynamics to drive neuritogenesis in embryonic cortical neurons.J Neurochem. 2022 Jan;160(2):185-202. doi: 10.1111/jnc.15502. Epub 2021 Sep 20. J Neurochem. 2022. PMID: 34478582
-
Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain.J Neurosci. 2006 Jun 21;26(25):6834-40. doi: 10.1523/JNEUROSCI.1728-06.2006. J Neurosci. 2006. PMID: 16793890 Free PMC article.
-
Drebrin in Neuronal Migration and Axonal Growth.Adv Exp Med Biol. 2017;1006:141-155. doi: 10.1007/978-4-431-56550-5_9. Adv Exp Med Biol. 2017. PMID: 28865019 Review.
-
General Introduction to Drebrin.Adv Exp Med Biol. 2017;1006:3-22. doi: 10.1007/978-4-431-56550-5_1. Adv Exp Med Biol. 2017. PMID: 28865011 Review.
Cited by
-
With the Permission of Microtubules: An Updated Overview on Microtubule Function During Axon Pathfinding.Front Mol Neurosci. 2021 Dec 2;14:759404. doi: 10.3389/fnmol.2021.759404. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34924953 Free PMC article. Review.
-
Drebrin Regulates Collateral Axon Branching in Cortical Layer II/III Somatosensory Neurons.J Neurosci. 2023 Nov 15;43(46):7745-7765. doi: 10.1523/JNEUROSCI.0553-23.2023. Epub 2023 Oct 5. J Neurosci. 2023. PMID: 37798130 Free PMC article.
-
Drebrin is Required for Myosin-facilitated Actin Cytoskeletal Remodeling during Pulmonary Alveolar Development.Am J Respir Cell Mol Biol. 2024 Apr;70(4):308-321. doi: 10.1165/rcmb.2023-0229OC. Am J Respir Cell Mol Biol. 2024. PMID: 38271699 Free PMC article.
-
PLK4 as a Key Regulator of Neuroblastoma Differentiation and a Promising Therapeutic Target.Int J Biol Sci. 2025 Jul 28;21(11):4979-4996. doi: 10.7150/ijbs.111449. eCollection 2025. Int J Biol Sci. 2025. PMID: 40860200 Free PMC article.
-
Drebrin Regulates Acetylcholine Receptor Clustering and Organization of Microtubules at the Postsynaptic Machinery.Int J Mol Sci. 2021 Aug 30;22(17):9387. doi: 10.3390/ijms22179387. Int J Mol Sci. 2021. PMID: 34502296 Free PMC article.
References
-
- Rakic P., Principles of neural cell migration. Experientia 46, 882–891 (1990). - PubMed
-
- Zou Y. R., Kottmann A. H., Kuroda M., Taniuchi I., Littman D. R., Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998). - PubMed
-
- Wray S., Development of gonadotropin-releasing hormone-1 neurons. Front. Neuroendocrinol. 23, 292–316 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

