Lenalidomide versus bortezomib maintenance after frontline autologous stem cell transplantation for multiple myeloma
- PMID: 33414374
- PMCID: PMC7791127
- DOI: 10.1038/s41408-020-00390-3
Lenalidomide versus bortezomib maintenance after frontline autologous stem cell transplantation for multiple myeloma
Abstract
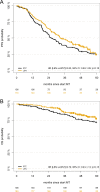
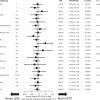
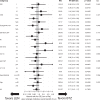
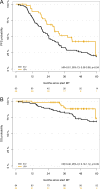
Lenalidomide (LEN) maintenance (MT) post autologous stem cell transplantation (ASCT) is standard of care in newly diagnosed multiple myeloma (MM) but has not been compared to other agents in clinical trials. We retrospectively compared bortezomib (BTZ; n = 138) or LEN (n = 183) MT from two subsequent GMMG phase III trials. All patients received three cycles of BTZ-based triplet induction and post-ASCT MT. BTZ MT (1.3 mg/m2 i.v.) was administered every 2 weeks for 2 years. LEN MT included two consolidation cycles (25 mg p.o., days 1-21 of 28 day cycles) followed by 10-15 mg/day for 2 years. The BTZ cohort more frequently received tandem ASCT (91% vs. 33%) due to different tandem ASCT strategies. In the LEN and BTZ cohort, 43% and 46% of patients completed 2 years of MT as intended (p = 0.57). Progression-free survival (PFS; HR = 0.83, p = 0.18) and overall survival (OS; HR = 0.70, p = 0.15) did not differ significantly with LEN vs. BTZ MT. Patients with <nCR after first ASCT were assigned tandem ASCT in both trials. In patients with <nCR and tandem ASCT (LEN: n = 54 vs. BTZ: n = 84), LEN MT significantly improved PFS (HR = 0.61, p = 0.04) but not OS (HR = 0.46, p = 0.09). In conclusion, the significant PFS benefit after eliminating the impact of different tandem ASCT rates supports the current standard of LEN MT after ASCT.
Conflict of interest statement
MAB: Takeda: Consultancy, honoraria; Novartis: Consultancy, Research Funding; Celgene, Amgen, Janssen: Travel grants. EKM: honoraria: Janssen, Celgene, Takeda; consulting or advisory role: Janssen, Celgene, Takeda; research funding: Takeda; travel, accommodations, expenses: Janssen, Takeda, Celgene, Mundipharma. TH: no COI. UB: no COI. HJS: Amgen: Honoraria, Other: Travel or accommodations; Bristol-Myers Squibb: Honoraria, Other: Travel or accommodations; Janssen Cilag: Honoraria, Other: Travel or accommodations; Sanofi: Honoraria, Other: Travel or accommodations; Celgene: Honoraria, Other: Travel or accommodations; AbbVie: Honoraria; Takeda: Honoraria, Other: Travel or accommodations. MMu: honoraria: Janssen, BMS, Takeda, Celgene, Amgen; consulting or advisory role: Janssen, BMS, Takeda, Celgene, Amgen; research funding: BMS; travel, accomodations, expenses: Janssen, BMS, Takeda, Amgen. SF: Advisory board: BMS, Celgene und Amgen. UD: Alexion: Honoraria; Janssen: Honoraria. PB: consulting or advisory role: BMS, AMGEN, Roche, MSD; research funding: BMS; travel, accomodations, expenses: BMS. KN: no COI. JS: no COI. CK: no COI. MSR: honoraria: Celgene, BMS,Novartis, Janssen, Takeda; consulting or advisory role: Celgene, BMS,Novartis, Janssen, Takeda; research funding: Celgene, Novartis, AMGEN; travel, accomodations, expenses: Janssen, BMS, Takeda. JH: Advisory boards: Adaptive, Amgen, BMS, Celgene, GSK, Janssen, Oncotracker. AJ: no COI. AS: no COI. DH: no COI. SL: no COI. PS: SkylineDx: Membership on an entity’s Board of Directors or advisory committees; Karyopharm: Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. HL: Janssen: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Amgen: Honoraria. HM: no COI. MG: no COI. MHo: honoraria: MSD. HWL: no COI. HB: no COI. IWB: research funding: Celgene, BMS, Janssen. CS: honoraria: BMS, Janssen, Celgene, Novartis, Amgen, Takeda; consulting or advisory role: BMS, Janssen, Celgene, Novartis, Amgen, Takeda; speakers bureau: Takeda; research funding: Takeda, Novartis; travel, accomodations, expenses: BMS, Janssen, Celgene, Novartis, Amgen, Takeda. BB: no COI. KCW: honoraria: AMGEN, BMS, Celgene, Novartis, Janssen, Takeda; consulting or advisory role: AMGEN, BMS, Celgene, Juno, Janssen, Adaptive, Sanofi, Takeda; research funding: AMGEN, Celgene, Sanofi, Janssen. MHä: honoraria: Novartis, Amgen, Roche, Takeda; consulting or advisory role: Celgene. JD: consulting or advisory role: Celgene; speakers bureau: Celgene; travel, accomodations, expenses: Celgene. HG: honoraria: Amgen, BMS, Celgene, Chugai, Janssen, Novartis, Takeda; consulting or advisory role: Amgen, BMS, Celgene, Chugai, Janssen, Novartis, Takeda; speakers bureau: Amgen, BMS, Celgene, Janssen, Novartis, Takeda; research funding: Amgen, BMS, Celgene, Chugai, Janssen, Novartis, Takeda; travel, accomodations, expenses: BMS, Celgene, Janssen, Novartis, Takeda.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

