Targeting TGFβ signal transduction for cancer therapy
- PMID: 33414388
- PMCID: PMC7791126
- DOI: 10.1038/s41392-020-00436-9
Targeting TGFβ signal transduction for cancer therapy
Abstract
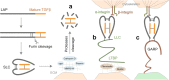
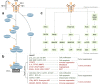
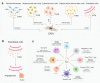
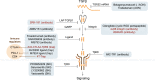
Transforming growth factor-β (TGFβ) family members are structurally and functionally related cytokines that have diverse effects on the regulation of cell fate during embryonic development and in the maintenance of adult tissue homeostasis. Dysregulation of TGFβ family signaling can lead to a plethora of developmental disorders and diseases, including cancer, immune dysfunction, and fibrosis. In this review, we focus on TGFβ, a well-characterized family member that has a dichotomous role in cancer progression, acting in early stages as a tumor suppressor and in late stages as a tumor promoter. The functions of TGFβ are not limited to the regulation of proliferation, differentiation, apoptosis, epithelial-mesenchymal transition, and metastasis of cancer cells. Recent reports have related TGFβ to effects on cells that are present in the tumor microenvironment through the stimulation of extracellular matrix deposition, promotion of angiogenesis, and suppression of the anti-tumor immune reaction. The pro-oncogenic roles of TGFβ have attracted considerable attention because their intervention provides a therapeutic approach for cancer patients. However, the critical function of TGFβ in maintaining tissue homeostasis makes targeting TGFβ a challenge. Here, we review the pleiotropic functions of TGFβ in cancer initiation and progression, summarize the recent clinical advancements regarding TGFβ signaling interventions for cancer treatment, and discuss the remaining challenges and opportunities related to targeting this pathway. We provide a perspective on synergistic therapies that combine anti-TGFβ therapy with cytotoxic chemotherapy, targeted therapy, radiotherapy, or immunotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

