Adiponectin signalling in bone homeostasis, with age and in disease
- PMID: 33414405
- PMCID: PMC7790832
- DOI: 10.1038/s41413-020-00122-0
Adiponectin signalling in bone homeostasis, with age and in disease
Abstract
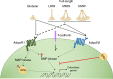
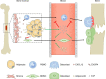
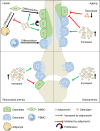
Adiponectin is the most abundant circulating adipokine and is primarily involved in glucose metabolism and insulin resistance. Within the bone, osteoblasts and osteoclasts express the adiponectin receptors, however, there are conflicting reports on the effects of adiponectin on bone formation and turnover. Many studies have shown a pro-osteogenic role for adiponectin in in vivo murine models and in vitro: with increased osteoblast differentiation and activity, alongside lower levels of osteoclastogenesis. However, human studies often demonstrate an inverse relationship between adiponectin concentration and bone activity. Moreover, the presence of multiple isoforms of adiponectin and multiple receptor subtypes has the potential to lead to more complex signalling and functional consequences. As such, we still do not fully understand the importance of the adiponectin signalling pathway in regulating bone homeostasis and repair in health, with age and in disease. In this review, we explore our current understanding of adiponectin bioactivity in the bone; the significance of its different isoforms; and how adiponectin biology is altered in disease. Ultimately, furthering our understanding of adiponectin regulation of bone biology is key to developing pharmacological and non-pharmacological (lifestyle) interventions that target adiponectin signalling to boost bone growth and repair in healthy ageing, following injury or in disease.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

