Incidence of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including CNS relapse in a population-based cohort of 4243 patients in Sweden
- PMID: 33414443
- PMCID: PMC7791057
- DOI: 10.1038/s41408-020-00403-1
Incidence of relapsed/refractory diffuse large B-cell lymphoma (DLBCL) including CNS relapse in a population-based cohort of 4243 patients in Sweden
Abstract
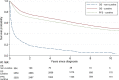
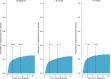
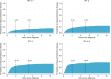
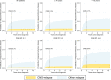
We performed a national population-based study of all patients diagnosed with diffuse large B-cell lymphoma (DLBCL) in Sweden in 2007-2014 to assess treatment intent and risk of relapsed/refractory disease, including central nervous system (CNS) relapse, in the presence of competing risks. Overall, 84% of patients started treatment with curative intent (anthracycline-based) (n = 3550, median age 69 years), whereas 14% did not (n = 594, median age 84 years) (for 2% the intent was uncertain). Patients treated with curative intent had a 5-year OS of 65.3% (95% CI: 63.7-66.9). The median OS among non-curatively treated patients was 2.9 months. The 5-year cumulative incidence of relapsed/refractory disease in curative patients was 23.1% (95% CI: 21.7-24.6, n = 847). The 2-year cumulative incidence of CNS relapse was 3.0% (95% CI: 2.5-3.6, n = 118) overall, and 8.0% (95% CI: 6.0-10.6, n = 48) among patients with high CNS-IPI (4-6), when considering other relapse locations and death as competing events. The incidence of relapsed/refractory DLBCL overall and in the CNS was lower than in previous reports, still one in seven patients was not considered fit enough to start standard immunochemotherapy at diagnosis. These results are important for quantification of groups of DLBCL patients with poor prognosis requiring completely different types of interventions.
Conflict of interest statement
This study was financed partly through the Swedish Cancer Society and partly through a public–private real-world evidence collaboration between Karolinska Institutet and Janssen Pharmaceutical NV. The funding bodies supported the data collection but did not have a role in the study design, data analyses or manuscript writing/decision to publish. Drs. Harrysson, Eloranta, Ekberg, Enblad, Jerkeman, Wahlin, Andersson, Smedby have nothing further to disclose.
Figures
References
-
- Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA Cancer J Clin. 2010;60:393–408. - PubMed
-
- Coiffier B, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. - DOI - PMC - PubMed
-
- Pfreundschuh M, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–22. doi: 10.1016/S1470-2045(11)70235-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

