A modular and controllable T cell therapy platform for acute myeloid leukemia
- PMID: 33414484
- PMCID: PMC7789085
- DOI: 10.1038/s41375-020-01109-w
A modular and controllable T cell therapy platform for acute myeloid leukemia
Abstract
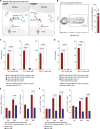
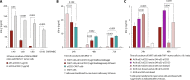
Targeted T cell therapy is highly effective in disease settings where tumor antigens are uniformly expressed on malignant cells and where off-tumor on-target-associated toxicity is manageable. Although acute myeloid leukemia (AML) has in principle been shown to be a T cell-sensitive disease by the graft-versus-leukemia activity of allogeneic stem cell transplantation, T cell therapy has so far failed in this setting. This is largely due to the lack of target structures both sufficiently selective and uniformly expressed on AML, causing unacceptable myeloid cell toxicity. To address this, we developed a modular and controllable MHC-unrestricted adoptive T cell therapy platform tailored to AML. This platform combines synthetic agonistic receptor (SAR) -transduced T cells with AML-targeting tandem single chain variable fragment (scFv) constructs. Construct exchange allows SAR T cells to be redirected toward alternative targets, a process enabled by the short half-life and controllability of these antibody fragments. Combining SAR-transduced T cells with the scFv constructs resulted in selective killing of CD33+ and CD123+ AML cell lines, as well as of patient-derived AML blasts. Durable responses and persistence of SAR-transduced T cells could also be demonstrated in AML xenograft models. Together these results warrant further translation of this novel platform for AML treatment.
© 2021. The Author(s).
Conflict of interest statement
SK, CK, and SE are inventors of several patents in the field of immuno-oncology including one patent application on the SAR platform. SK and SE received research support from TCR2 Inc and Arcus Bioscience for work unrelated to this manuscript. Parts of this work have been performed for the doctoral thesis of MRB at the Ludwig-Maximilians-Universität München. The authors declare no other conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

