Effect of gonadotropin-releasing hormone analog treatment on final height in girls aged 6-10 years with central precocious and early puberty
- PMID: 33414653
- PMCID: PMC7750341
- DOI: 10.14744/TurkPediatriArs.2020.01700
Effect of gonadotropin-releasing hormone analog treatment on final height in girls aged 6-10 years with central precocious and early puberty
Abstract
Aim: To determine the effects of gonadotropin-releasing hormone analog treatment on final height and body mass index in girls with central precocious puberty.
Material and methods: All cases with diagnosis age <8 years constituted group 1 (n=19) and those with ≥8 years constituted group 2 (n=35).
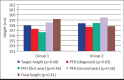
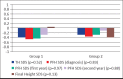
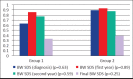
Results: There was no significant difference in height standard deviation score, body mass index standard deviation score, bone age/chronologic age, predicted final height at the time of diagnosis, and follow-up between group 1 and group 2. There was no significant difference in final height (standard deviation score) between the groups. The number of obese and overweight cases at diagnosis and final height was similar. The target height (standard deviation score), predicted final height (standard deviation score), and final height (standard deviation score) were similar in both Group 1 and Group 2.
Conclusion: We found that between the ages of 6-9.8 years, girls with central precocious puberty who received gonadotropin-releasing hormone analog treatment reached a final height within their target height range. It is concluded that gonadotropin-releasing hormone analog treatment increases body mass index during treatment and when patients reach the final height, they return to their pretreatment body mass index. Younger age and greater height at the time of diagnosis are the positive factors on final height.
Amaç: Santral erken puberteli olgularda Gonadotropin Releasing Hormon agonist tedavisinin final boy ve vücut kitle indeksi üzerine etkisinin değerlendirilmesini amaçladık.
Gereç ve yöntemler: Çalışmaya alınan 54 kız hasta tanı yaşlarına göre 2 gruba ayrıldı. Tanı yaşı 8 yaş altı olan olgular Grup 1’i, 8 yaş ve üzeri olan olgular Grup 2’yi oluşturdu. Grup 1’de 19 hasta, Grup 2’de 35 hasta vardı.
Bulgular: Tanıda ve izlem yıllarında boy standart deviasyon skoru, vücut kitle indeksi standart deviasyon skoru, kemik yaşı/takvim yaşı, öngörülen son boy açısından Grup 1 ile Grup 2 arasında istatistiksel olarak anlamlı fark yoktu. Gruplar arasında final boy, final boy standart deviasyon skoru açısından fark yoktu. Tanıda ve finalde obez ve kilolu olgu sayısı benzerdi. Hem Grup 1 hem Grup 2’de hedef boy, öngörülen son boy ve final boylar arasında ve bunların standart deviasyon skorları arasında istatistiksel olarak anlamlı fark saptanmadı.
Çikarimlar: Bu çalışmada Gonadotropin Releasing Hormon agonist tedavisi verilen 6–9.8 yaş aralığındaki santral erken puberteli kızların hedef boyları ile uyumlu final boya ulaştıkları belirlendi. Gonadotropin Releasing Hormon agonist tedavisinin tedavi sırasında vücut kitle indeksini arttırdığı, tedavi kesiminden sonra final boya ulaşıldığında hastaların tanı vücut kitle indekslerine geri döndükleri sonucuna varıldı. Tanı yaşının küçük olması ve tanı boyunun uzun olması final boy üzerine pozitif etkili etmenler olduğu bulundu.
Keywords: Central precocious puberty; early puberty; final height; gonadotropin-releasing hormone analog treatment; rapidly progressive puberty.
Copyright: © 2020 Turkish Archives of Pediatrics.
Conflict of interest statement
Conflict of Interest: The authors have no conflicts of interest to declare.
Figures
Similar articles
-
[Effect of treatment with gonadotropin releasing hormone analogues in girls with idiopathic central precocious puberty].Orv Hetil. 2012 Mar 18;153(11):418-24. doi: 10.1556/OH.2012.29320. Orv Hetil. 2012. PMID: 22390866 Hungarian.
-
The Gonadotropin-Releasing Hormone Analogue Therapy May Not Impact Final Height in Precocious Puberty of Girls With Onset of Puberty Aged 6 - 8 Years.J Clin Med Res. 2019 Feb;11(2):133-136. doi: 10.14740/jocmr3710. Epub 2019 Jan 5. J Clin Med Res. 2019. PMID: 30701006 Free PMC article.
-
Retrospective evaluation of patients diagnosed with central precocious puberty who reached the final height.J Pediatr Endocrinol Metab. 2024 Jun 18;37(8):715-721. doi: 10.1515/jpem-2024-0124. Print 2024 Aug 27. J Pediatr Endocrinol Metab. 2024. PMID: 38881279
-
Gonadotropin releasing hormone agonist treatment to increase final stature in children with precocious puberty: a meta-analysis.Medicine (Baltimore). 2014 Dec;93(27):e260. doi: 10.1097/MD.0000000000000260. Medicine (Baltimore). 2014. PMID: 25501098 Free PMC article. Review.
-
Toward More Targeted and Cost-Effective Gonadotropin-Releasing Hormone Analog Treatment in Girls with Central Precocious Puberty.Horm Res Paediatr. 2018;90(1):1-7. doi: 10.1159/000491103. Epub 2018 Jul 26. Horm Res Paediatr. 2018. PMID: 30048994 Review.
Cited by
-
Pharmacotherapy for children with central precocious puberty or early puberty: A systematic review and meta-analysis.Medicine (Baltimore). 2025 Aug 1;104(31):e41936. doi: 10.1097/MD.0000000000041936. Medicine (Baltimore). 2025. PMID: 40760548 Free PMC article.
-
The Effect of GnRHa Treatment on Body Mass Index in Central Precocious Puberty: A Systematic Review and Meta-Analysis.Horm Res Paediatr. 2024;97(5):419-432. doi: 10.1159/000535132. Epub 2024 Jan 5. Horm Res Paediatr. 2024. PMID: 38185120 Free PMC article.
-
Long-Term Efficacy and Safety of Leuprorelin Treatment in Children with Central Precocious Puberty: A Systematic Review and Meta-Analysis.Children (Basel). 2025 May 30;12(6):712. doi: 10.3390/children12060712. Children (Basel). 2025. PMID: 40564670 Free PMC article. Review.
-
Early Normal Puberty and Accelerated Puberty in Girls: How Can We Avoid Unnecessary Treatment and Identify Children Who Are Likely to Benefit from Gonadotropin-Releasing Hormone Agonist Treatment?Turk Arch Pediatr. 2023 Nov;58(6):664-667. doi: 10.5152/TurkArchPediatr.2023.23104. Turk Arch Pediatr. 2023. PMID: 37553970 Free PMC article. No abstract available.
-
The Effect of GnRH Analogs on Body Mass Index in Girls with Central Precocious Puberty: A Single-Center Retrospective Study with a Literature Review.Children (Basel). 2025 Mar 7;12(3):336. doi: 10.3390/children12030336. Children (Basel). 2025. PMID: 40150620 Free PMC article.
References
-
- Lee PA, Houk CP. Puberty and Its Disorders. In: Liftshitz F, editor. Pediatric Endocrinology. New York, USA: Informa Healthcare Inc; 2007. pp. 274–90.
-
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity:variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. - PubMed
-
- Klein KO, Barnes KM, Jones JV, Feuillan PP, Cutler GB., Jr Increased final height in precocious puberty after long-term treatment with LHRH agonists:the National Institutes of Health experience. J Clin Endocrinol Metab. 2001;86:4711–6. - PubMed
-
- Lazar L, Padoa A, Phillip M. Growth pattern and final height after cessation of gonadotropin-suppressive therapy in girls with central sexual precocity. J Clin Endocrinol Metab. 2007;92:3483–9. - PubMed
LinkOut - more resources
Full Text Sources