Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms
- PMID: 33414705
- PMCID: PMC7783407
- DOI: 10.3389/fnins.2020.619667
Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms
Abstract
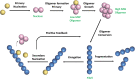
Alzheimer's disease (AD) is one of the most common neurodegenerative disorders, with no cure and preventive therapy. Misfolding and extracellular aggregation of Amyloid-β (Aβ) peptides are recognized as the main cause of AD progression, leading to the formation of toxic Aβ oligomers and to the deposition of β-amyloid plaques in the brain, representing the hallmarks of AD. Given the urgent need to provide alternative therapies, natural products serve as vital resources for novel drugs. In recent years, several natural compounds with different chemical structures, such as polyphenols, alkaloids, terpenes, flavonoids, tannins, saponins and vitamins from plants have received attention for their role against the neurodegenerative pathological processes. However, only for a small subset of them experimental evidences are provided on their mechanism of action. This review focuses on those natural compounds shown to interfere with Aβ aggregation by direct interaction with Aβ peptide and whose inhibitory mechanism has been investigated by means of biophysical and structural biology experimental approaches. In few cases, the combination of approaches offering a macroscopic characterization of the oligomers, such as TEM, AFM, fluorescence, together with high-resolution methods could shed light on the complex mechanism of inhibition. In particular, solution NMR spectroscopy, through peptide-based and ligand-based observation, was successfully employed to investigate the interactions of the natural compounds with both soluble NMR-visible (monomer and low molecular weight oligomers) and NMR-invisible (high molecular weight oligomers and protofibrils) species. The molecular determinants of the interaction of promising natural compounds are here compared to infer the chemical requirements of the inhibitors and the common mechanisms of inhibition. Most of the data converge to indicate that the Aβ regions relevant to perturb the aggregation cascade and regulate the toxicity of the stabilized oligomers, are the N-term and β1 region. The ability of the natural aggregation inhibitors to cross the brain blood barrier, together with the tactics to improve their low bioavailability are discussed. The analysis of the data ensemble can provide a rationale for the selection of natural compounds as molecular scaffolds for the design of new therapeutic strategies against the progression of early and late stages of AD.
Keywords: Alzheimer; NMR; amyloid-β protein; natural compound; protein ligand interactions; self-association.
Copyright © 2020 Pagano, Tomaselli, Molinari and Ragona.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The ongoing search for small molecules to study metal-associated amyloid-β species in Alzheimer's disease.Acc Chem Res. 2014 Aug 19;47(8):2475-82. doi: 10.1021/ar500152x. Epub 2014 Jul 31. Acc Chem Res. 2014. PMID: 25080056
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
A Systematic Review on the Role of Natural Products in Modulating the Pathways in Alzheimer's Disease.Int J Vitam Nutr Res. 2017 Mar;87(1-2):99-116. doi: 10.1024/0300-9831/a000405. Epub 2018 Jul 16. Int J Vitam Nutr Res. 2017. PMID: 30010515
-
Natural compounds against Alzheimer's disease: molecular recognition of Aβ1-42 peptide by Salvia sclareoides extract and its major component, rosmarinic acid, as investigated by NMR.Chem Asian J. 2013 Mar;8(3):596-602. doi: 10.1002/asia.201201063. Epub 2013 Jan 9. Chem Asian J. 2013. PMID: 23303581
-
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer's Disease and Other Amyloid Diseases.Acc Chem Res. 2018 Mar 20;51(3):706-718. doi: 10.1021/acs.accounts.7b00554. Epub 2018 Mar 6. Acc Chem Res. 2018. PMID: 29508987 Free PMC article. Review.
Cited by
-
Glucosyl Platinum(II) Complexes Inhibit Aggregation of the C-Terminal Region of the Aβ Peptide.Inorg Chem. 2022 Feb 28;61(8):3540-3552. doi: 10.1021/acs.inorgchem.1c03540. Epub 2022 Feb 16. Inorg Chem. 2022. PMID: 35171608 Free PMC article.
-
Chiral Fibers Formation Upon Assembly of Tetraphenylalanine Peptide Conjugated to a PNA Dimer.Chemistry. 2022 Jul 1;28(37):e202200693. doi: 10.1002/chem.202200693. Epub 2022 May 23. Chemistry. 2022. PMID: 35474351 Free PMC article.
-
Phytochemical Interactions with Calmodulin and Critical Calmodulin Binding Proteins Involved in Amyloidogenesis in Alzheimer's Disease.Biomolecules. 2023 Apr 15;13(4):678. doi: 10.3390/biom13040678. Biomolecules. 2023. PMID: 37189425 Free PMC article. Review.
-
Azepine-Indole Alkaloids From Psychotria nemorosa Modulate 5-HT2A Receptors and Prevent in vivo Protein Toxicity in Transgenic Caenorhabditis elegans.Front Neurosci. 2022 Mar 14;16:826289. doi: 10.3389/fnins.2022.826289. eCollection 2022. Front Neurosci. 2022. PMID: 35360162 Free PMC article.
-
Exploring the Benzazoles Derivatives as Pharmacophores for AChE, BACE1, and as Anti-Aβ Aggregation to Find Multitarget Compounds against Alzheimer's Disease.Molecules. 2024 Oct 9;29(19):4780. doi: 10.3390/molecules29194780. Molecules. 2024. PMID: 39407708 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

