Blood-Brain Barrier Leakage Is Increased in Parkinson's Disease
- PMID: 33414722
- PMCID: PMC7784911
- DOI: 10.3389/fphys.2020.593026
Blood-Brain Barrier Leakage Is Increased in Parkinson's Disease
Abstract
Background: Blood-brain barrier (BBB) disruption has been noted in animal models of Parkinson's disease (PD) and forms the basis of the vascular hypothesis of neurodegeneration, yet clinical studies are lacking.
Objective: To determine alterations in BBB integrity in PD, with comparison to cerebrovascular disease.
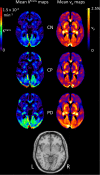
Methods: Dynamic contrast enhanced magnetic resonance images were collected from 49 PD patients, 15 control subjects with cerebrovascular disease [control positive (CP)] and 31 healthy control subjects [control negative (CN)], with all groups matched for age. Quantitative maps of the contrast agent transfer coefficient across the BBB (K trans) and plasma volume (v p ) were produced using Patlak analysis. Differences in K trans and v p were assessed with voxel-based analysis as well as in regions associated with PD pathophysiology. In addition, the volume of white matter lesions (WMLs) was obtained from T2-weighted fluid attenuation inversion recovery (FLAIR) images.
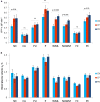
Results: Higher K trans, reflecting higher BBB leakage, was found in the PD group than in the CN group using voxel-based analysis; differences were most prominent in the posterior white matter regions. Region of interest analysis confirmed K trans to be significantly higher in PD than in CN, predominantly driven by differences in the substantia nigra, normal-appearing white matter, WML and the posterior cortex. WML volume was significantly higher in PD compared to CN. K trans values and WML volume were similar in PD and CP, suggesting a similar burden of cerebrovascular disease despite lower cardiovascular risk factors.
Conclusion: These results show BBB disruption in PD.
Keywords: Parkinson’s disease; blood–brain barrier; cerebrovascular disease; dynamic contrast enhanced MRI; neurovascular unit.
Copyright © 2020 Al-Bachari, Naish, Parker, Emsley and Parkes.
Conflict of interest statement
Authors JN and GP were part employed by the company Bioxydyn and hold shares in the company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

